Page 80 - Williams Hematology ( PDFDrive )
P. 80
56 Part II: The Organization of the Lymphohematopoietic Tissues Chapter 5: Structure of the Marrow and the Hematopoietic Microenvironment 57
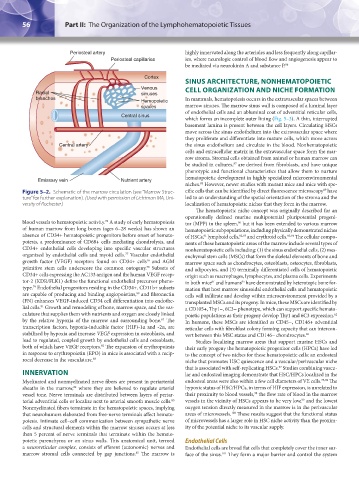
Periosteal artery highly innervated along the arterioles and less frequently along capillar-
Periosteal capillaries ies, where neurologic control of blood flow and angiogenesis appear to
be mediated via neurokinin A and substance P. 86
Cortex
SINUS ARCHITECTURE, NONHEMATOPOIETIC
Venous CELL ORGANIZATION AND NICHE FORMATION
Radial sinuses
branches In mammals, hematopoiesis occurs in the extravascular spaces between
Hemopoietic
spaces marrow sinuses. The marrow sinus wall is composed of a luminal layer
of endothelial cells and an abluminal coat of adventitial reticular cells,
Central sinus
which forms an incomplete outer lining (Fig. 5–3). A thin, interrupted
basement lamina is present between the cell layers. Circulating HSCs
move across the sinus endothelium into the extravascular space where
they proliferate and differentiate into mature cells, which move across
Central artery the sinus endothelium and circulate in the blood. Nonhematopoietic
cells and extracellular matrix in the extravascular space form the mar-
row stroma. Stromal cells obtained from animal or human marrow can
87
be studied in cultures, are derived from fibroblasts, and have unique
phenotypic and functional characteristics that allow them to nurture
hematopoietic development in highly specialized microenvironmental
Emissary vein Nutrient artery
niches. However, newer studies with mutant mice and mice with spe-
88
89
Figure 5–2. Schematic of the marrow circulation (see “Marrow Struc- cific cells that can be identified by direct fluorescence microscopy have
ture” for further explanation). (Used with permission of Lichtman MA, Uni- led to an understanding of the spatial orientation of the stroma and the
versity of Rochester.) localization of hematopoietic niches that they form in the marrow.
The hematopoietic niche concept was originally described for an
operationally defined murine multipotential pluripotential progeni-
blood vessels to hematopoietic activity. A study of early hematopoiesis tor (MPP) in the spleen, but it has been extended to various marrow
76
90
of human marrow from long bones (ages 6–28 weeks) has shown an hematopoietic subpopulations, including physically demonstrated niches
absence of CD34+ hematopoietic progenitors before onset of hemato- of HSCs, lymphoid cells, 92,93 and erythroid cells. 93,94 The cellular compo-
91
poiesis, a predominance of CD68+ cells mediating chondrolysis, and nents of these hematopoietic areas of the marrow include several types of
CD34+ endothelial cells developing into specific vascular structures nonhematopoietic cells including: (1) the sinus endothelial cells, (2) mes-
organized by endothelial cells and myoid cells. Vascular endothelial enchymal stem cells (MSCs) that form the skeletal elements of bone and
77
growth factor (VEGF) receptors found on CD34+ cells and AGM marrow space such as chondrocytes, osteoblasts, osteocytes, fibroblasts,
16
primitive stem cells underscore the common ontogeny. Subsets of and adipocytes, and (3) terminally differentiated cells of hematopoietic
78
CD34+ cells expressing the AC133 antigen and the human VEGF recep- origin such as macrophages, lymphocytes, and plasma cells. Experiments
tor-2 (KDR/FLK1) define the functional endothelial precursor pheno- in both mice and humans have demonstrated by heterotopic bone for-
95
61
type. Endothelial progenitors residing in the CD34+, CD11b+ subsets mation that host marrow sinusoidal endothelial cells and hematopoietic
79
are capable of producing and binding angiopoietins, and fibronectin cells will infiltrate and develop within microenvironment provided by a
80
(FN) enhances VEGF-induced CD34 cell differentiation into endothe- transplanted MSCs and its progeny. In mice, these MSCs are identified by
lial cells. Growth and remodeling of bone, marrow space, and the vas- a CD105+, Thy1−, 6C3− phenotype, which can support specific hemato-
81
culature that supplies them with nutrients and oxygen are closely linked poietic populations as their progeny develop Thy1 and 6C3 expression.
62
by the relative hypoxia of the marrow and surrounding bone. The In humans, these MSCs are identified as CD45−, CD146+ adventitial
82
transcription factors, hypoxia-inducible factor (HIF)-1α and -2α, are reticular cells with fibroblast colony forming capacity that can intercon-
stabilized by hypoxia and increase VEGF expression in osteoblasts, and vert between this MSC status and CD146− chondrocytes. 96
lead to regulated, coupled growth by endothelial cells and osteoblasts, Studies localizing marrow areas that support murine HSCs and
both of which have VEGF receptors. The expansion of erythropoiesis their early progeny the hematopoietic progenitor cells (HPCs) have led
82
in response to erythropoietin (EPO) in mice is associated with a recip- to the concept of two niches for these hematopoietic cells: an endosteal
rocal decrease in the vasculature. 83 niche that promotes HSC quiescence and a vascular/perivascular niche
97
that is associated with self-replicating HSCs. Studies combining vascu-
INNERVATION lar and endosteal imaging demonstrate that HSC/HPCs localized in the
Myelinated and nonmyelinated nerve fibers are present in periarterial endosteal areas were also within a few cell diameters of VE cells. 75,98 The
sheaths in the marrow, where they are believed to regulate arterial hypoxic status of HSC/HPCs, in terms of HIF expression, is unrelated to
84
98
vessel tone. Nerve terminals are distributed between layers of periar- their proximity to blood vessels, the flow rate of blood in the marrow
99
terial adventitial cells or localize next to arterial smooth muscle cells. vessels in the vicinity of HSCs appears to be very low, and the lowest
85
Nonmyelinated fibers terminate in the hematopoietic spaces, implying oxygen tension directly measured in the marrow is in the perivascular
that neurohumors elaborated from free-nerve terminals affect hemato- areas of microvessels. These results suggest that the functional status
100
poiesis. Intimate cell–cell communication between sympathetic nerve of microvessels has a larger role in HSC niche activity than the proxim-
cells and structural elements within the marrow sinuses occurs at less ity of the potential niche to its vascular supply.
than 5 percent of nerve terminals that terminate within the hemato-
poietic parenchyma or on sinus walls. This anatomical unit, termed Endothelial Cells
a neuroreticular complex, consists of efferent (autonomic) nerves and Endothelial cells are broad flat cells that completely cover the inner sur-
marrow stromal cells connected by gap junctions. The marrow is face of the sinus. They form a major barrier and control the system
85
101
Kaushansky_chapter 05_p0051-0084.indd 56 9/19/15 12:10 AM