Page 1096 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1096
1060 ParT EighT Immunology of Neoplasia
TABLE 78.5 Major Factors associated With Prognosis of Chronic Lymphocytic
Leukemia (CLL) 30,4,5,6,7,9,31,33,34
Median Used in Clinical
Definition Survival (Years) Practice
Rai stage 0 Leukocytosis 12.5 Yes
1 Leukocytosis and lymphadenopathy 8.4
2 Lymphocytosis plus hepatosplenomegaly 6.9
3 Lymphocytosis plus anemia (<11 gr/dL) 1.5
4 Lymphocytosis plus thrombocytopenia (<100 000 × 10 /L) 1.5
9
Binet Leukocytosis and lymphadenopathy Age-matched Yes
Stage A Lymphadenopathy of more than two involved areas 7
Stage B Anemia or thrombocytopenia 2
Stage C
β 2 microglobulin Normal 9.7 Yes
Elevated 4.5
CD 38 >30% 2.9 to <10 years Yes
Early-stage CLL <30% 9 to >26 years
Chromosomal aberrations 17p- 2.7 Sometimes
(FISH analysis) 11q- 6.5
Trisomy 12 9.5
Normal karyotype 9.3
13q- 11.1
Zeta-associated protein 70 (ZAP-70) >20% 7.5 If available
Early-stage CLL <20% Not reached
Mutational status Unmutated 5.7 to <9.9 years If available
Mutated 10.2 to >24 years
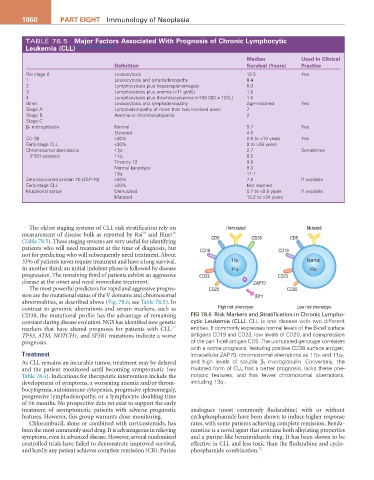
The oldest staging systems of CLL risk stratification rely on Unmutated Mutated
30
29
measurement of disease bulk as reported by Rai and Binet CD5 CD38 CD5
(Table 78.5). These staging systems are very useful for identifying
patients who will need treatment at the time of diagnosis, but CD19 CD19
not for predicting who will subsequently need treatment. About
33% of patients never require treatment and have a long survival. 17p- Normal
In another third, an initial indolent phase is followed by disease 11q- 13q-
progression. The remaining third of patients exhibit an aggressive CD23 CD23
disease at the onset and need immediate treatment. ZAP70
The most powerful predictors for rapid and aggressive progres- CD20 CD20
sion are the mutational status of the V domains and chromosomal β2m
abnormalities, as described above (Fig. 78.6; see Table 78.5). In
contrast to genomic aberrations and serum markers, such as High risk phenotype Low risk phenotype
CD38, the mutational profile has the advantage of remaining Fig 78.6 Risk Markers and Stratification in Chronic Lympho-
constant during disease evolution. NGS has identified new genetic cytic Leukemia (CLL). CLL is one disease with two different
31
markers that have altered prognosis for patients with CLL. entities. It commonly expresses normal levels of the B-cell surface
TP53, ATM, NOTCH1, and SF3B1 mutations indicate a worse antigens CD19 and CD23, low levels of CD20, and coexpression
prognosis. of the pan T-cell antigen CD5. The unmutated genotype correlates
with a worse prognosis, featuring positive CD38 surface antigen,
Treatment intracellular ZAP70, chromosomal aberrations as 17p- and 11q-,
As CLL remains an incurable tumor, treatment may be delayed and high levels of soluble β 2 microglobulin. Conversely, the
and the patient monitored until becoming symptomatic (see mutated form of CLL has a better prognosis, lacks these phe-
Table 78.4). Indications for therapeutic intervention include the notypic features, and has fewer chromosomal aberrations,
development of symptoms, a worsening anemia and/or throm- including 13q-.
bocytopenia, autoimmune cytopenias, progressive splenomegaly,
progressive lymphadenopathy, or a lymphocyte doubling time
of ≤6 months. No prospective data yet exist to support the early
treatment of asymptomatic patients with adverse prognostic analogues (most commonly fludarabine) with or without
features. However, this group warrants close monitoring. cyclophosphamide have been shown to induce higher response
Chlorambucil, alone or combined with corticosteroids, has rates, with some patients achieving complete remission. Benda-
been the most commonly used drug. It is advantageous in relieving mustine is a novel agent that contains both alkylating properties
symptoms, even in advanced disease. However, several randomized and a purine-like benzimidazole ring. It has been shown to be
controlled trials have failed to demonstrate improved survival, effective in CLL and less toxic than the fludarabine and cyclo-
and hardly any patient achieves complete remission (CR). Purine phosphamide combination. 32