Page 1094 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1094
1058 ParT EighT Immunology of Neoplasia
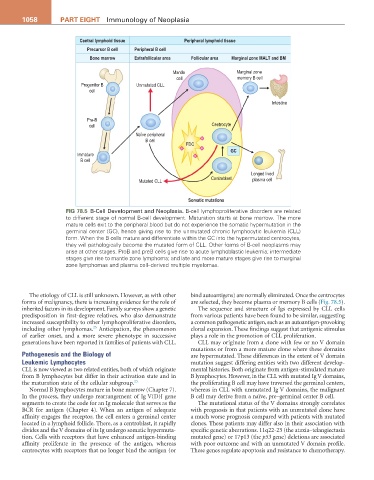
Central lymphoid tissue Peripheral lymphoid tissue
Precursor B cell Peripheral B cell
Bone marrow Extrafollicular area Follicular area Marginal zone MALT and BM
Mantle Marginal zone
cell memory B cell
Progenitor B Unmutated CLL
cell
Intestine
Pre-B
cell Centrocyte
Naïve peripheral
B cell
FDC
GC
Immature
B cell
Longed lived
Centroblast plasma cell
Mutated CLL
Somatic mutations
Fig 78.5 B-Cell Development and Neoplasia. B-cell lymphoproliferative disorders are related
to different stage of normal B-cell development. Maturation starts at bone marrow. The more
mature cells exit to the peripheral blood but do not experience the somatic hypermutation in the
germinal center (GC), hence giving rise to the unmutated chronic lymphocytic leukemia (CLL)
form. When the B cells mature and differentiate within the GC into the hypermutated centrocytes,
they will pathologically become the mutated form of CLL. Other forms of B-cell neoplasms may
arise at other stages. ProB and preB cells give rise to acute lymphoblastic leukemia; intermediate
stages give rise to mantle zone lymphoma; and late and more mature stages give rise to marginal
zone lymphomas and plasma cell–derived multiple myelomas.
The etiology of CLL is still unknown. However, as with other bind autoantigens) are normally eliminated. Once the centrocytes
forms of malignancy, there is increasing evidence for the role of are selected, they become plasma or memory B cells (Fig. 78.5).
inherited factors in its development. Family surveys show a genetic The sequence and structure of Igs expressed by CLL cells
predisposition in first-degree relatives, who also demonstrate from various patients have been found to be similar, suggesting
increased susceptibility to other lymphoproliferative disorders, a common pathogenetic antigen, such as an autoantigen-provoking
25
including other lymphomas. Anticipation, the phenomenon clonal expansion. These findings suggest that antigenic stimulus
of earlier onset, and a more severe phenotype in successive plays a role in the promotion of CLL proliferation.
generations have been reported in families of patients with CLL. CLL may originate from a clone with few or no V domain
mutations or from a more mature clone where these domains
Pathogenesis and the Biology of are hypermutated. These differences in the extent of V domain
Leukemic Lymphocytes mutation suggest differing entities with two different develop-
CLL is now viewed as two related entities, both of which originate mental histories. Both originate from antigen-stimulated mature
from B lymphocytes but differ in their activation state and in B lymphocytes. However, in the CLL with mutated Ig V domains,
the maturation state of the cellular subgroup. 23. the proliferating B cell may have traversed the germinal centers,
Normal B lymphocytes mature in bone marrow (Chapter 7). whereas in CLL with unmutated Ig V domains, the malignant
In the process, they undergo rearrangement of Ig V(D)J gene B cell may derive from a naïve, pre–germinal center B cell.
segments to create the code for an Ig molecule that serves as the The mutational status of the V domains strongly correlates
BCR for antigen (Chapter 4). When an antigen of adequate with prognosis in that patients with an unmutated clone have
affinity engages the receptor, the cell enters a germinal center a much worse prognosis compared with patients with mutated
located in a lymphoid follicle. There, as a centroblast, it rapidly clones. These patients may differ also in their association with
divides and the V domains of its Ig undergo somatic hypermuta- specific genetic aberrations. 11q22-23 (the ataxia–telangiectasia
tion. Cells with receptors that have enhanced antigen-binding mutated gene) or 17p13 (the p53 gene) deletions are associated
affinity proliferate in the presence of the antigen, whereas with poor outcome and with an unmutated V domain profile.
centrocytes with receptors that no longer bind the antigen (or These genes regulate apoptosis and resistance to chemotherapy.