Page 1169 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1169
CHAPTER 83 Hematopoietic Stem Cell Transplantation for Malignant Diseases 1133
TABLE 83.2 Staging of Chronic Graft-Versus-Host Disease (GvHD)
Target Organ Score 0 Score 1 Score 2 Score 3
Performance score KPS 100% KPS 80–90% KPS 60–70% KPS <60%
Skin No symptoms <18% BSA 19–50% or sclerotic, still able to >50% or “Hidebound”
pinch
Mouth No symptoms Mild symptoms, no Moderate symptoms, decreased Severe symptoms with
limitations oral intake major decrease in intake
Eyes No symptoms Mild dry eyes Moderate dry eyes, drops >3×/day Severe dry eyes affecting
daily activities
Gastrointestinal tract No symptoms Symptoms without Symptoms with moderate weight Symptoms with weight loss
weight loss loss (5–15%) >15%
Liver Normal LFTs LFTs elevated <2× upper LFTs elevated 2–5× upper limits LFTs elevated >5× upper
limits of normal of normal limits of normal
Lungs No symptoms Mild symptoms Moderate symptoms Severe symptoms
FEV 60–79% FEV 40–59% FEV <40%
Joints and fascia No symptoms Mild tightness not Tightness affecting daily activities Contractures with significant
affecting daily activities loss of range of motion
Female genital tract No symptoms Symptomatic with middle Symptomatic with dyspareunia Symptomatic with strictures
signs on examination
At least one diagnostic and one distinctive sign is necessary to make a diagnosis of cGvHD.
BSA, Body Surface Area; FEV, Forced Expiratory Volume; KPS, Karnofsky Performance Status; LFT, Liver Function Test.
Adapted from: Filipovitch AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host
Disease: 1. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant 2005; 11: 945–56.
CLINICAL PEARLS GvHD GvT
Factors Predicting Chronic Graft-Versus-Host
Disease (GvHD)
• Degree of human leukocyte antigen (HLA) incompatibility Target tissue- Shared alloantigens: Bcr-abl
• Major histocompatibility complex (MHC) restricted miHA MHC, miHA hematopoietic-restricted
• Minor histocompatibility antigens (miHAs) disparity miHA proteinase-3 c-akt
• Presence of prior acute GvHD
• Source of stem cells (higher risk in peripheral blood versus bone
marrow)
• Donor gender (female donor → male recipient)
• Use of donor lymphocyte infusion (DLI) following hematopoietic stem
cell transplantation (HSCT)
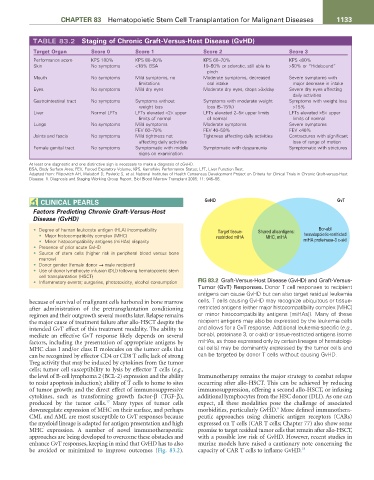
• Inflammatory events; surgeries, phototoxicity, alcohol consumption FIG 83.2 Graft-Versus-Host Disease (GvHD) and Graft-Versus-
Tumor (GvT) Responses. Donor T cell responses to recipient
antigens can cause GvHD but can also target residual leukemia
because of survival of malignant cells harbored in bone marrow cells. T cells causing GvHD may recognize ubiquitous or tissue-
after administration of the pretransplantation conditioning restricted antigens (either major histocompatibility complex [MHC]
regimen and their outgrowth several months later. Relapse remains or minor histocompatibility antigens [miHAs]). Many of these
the major cause of treatment failure after allo-HSCT despite the recipient antigens may also be expressed by the leukemia cells
intended GvT effect of this treatment modality. The ability to and allows for a GvT response. Additional leukemia-specific (e.g.,
mediate an effective GvT response likely depends on several bcr-abl, proteinase 3, or c-akt) or tissue-restricted antigens (some
factors, including the presentation of appropriate antigens by miHAs, as those expressed only by certain lineages of hematologi-
MHC class I and/or class II molecules on the tumor cells that cal cells) may be dominantly expressed by the tumor cells and
can be recognized by effector CD4 or CD8 T cells; lack of strong can be targeted by donor T cells without causing GvHD.
Treg activity that may be induced by cytokines from the tumor
cells; tumor cell susceptibility to lysis by effector T cells (e.g.,
the level of B-cell lymphoma 2 (BCL-2) expression and the ability Immunotherapy remains the major strategy to combat relapse
to resist apoptosis induction); ability of T cells to home to sites occurring after allo-HSCT. This can be achieved by reducing
of tumor growth; and the direct effect of immunosuppressive immunosuppression, offering a second allo-HSCT, or infusing
cytokines, such as transforming growth factor-β (TGF-β), additional lymphocytes from the HSC donor (DLI). As one can
17
produced by the tumor cells. Many types of tumor cells expect, all these modalities pose the challenge of associated
6
downregulate expression of MHC on their surface, and perhaps morbidities, particularly GvHD. More defined immunothera-
CML and AML are most susceptible to GvT responses because peutic approaches using chimeric antigen receptors (CARs)
the myeloid lineage is adapted for antigen presentation and high expressed on T cells (CAR T cells; Chapter 77) also show some
MHC expression. A number of novel immunotherapeutic promise to target residual tumor cells that remain after allo-HSCT,
approaches are being developed to overcome these obstacles and with a possible low risk of GvHD. However, recent studies in
enhance GvT responses, keeping in mind that GvHD has to also murine models have raised a cautionary note concerning the
be avoided or minimized to improve outcomes (Fig. 83.2). capacity of CAR T cells to inflame GvHD. 18