Page 1167 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1167
CHAPTER 83 Hematopoietic Stem Cell Transplantation for Malignant Diseases 1131
KEY CONCEPTS
Irradiation Chemotherapy DC Donor cells
Graft-Versus-Host Disease (GvHD)
DC/Mø
• Caused by donor–recipient differences in:
IL-12 • Major histocompatibility complex (MHC) molecules
• Minor histocompatibility antigens (miHAs)
• Mediated by mature donor CD4 and/or CD8 T cells
• Requires inflammatory cytokines
TNFα, IL-1
CD4 NK • Primary target organs include lymphoid system, skin, gastrointestinal
tract, and liver
Upregulates adhesion • Acute and chronic forms
molecules on endothelium Activation and CD8
and chemokines proliferation
IFNγ, TNFα
IL-2 may also be additional tumor-specific or hematopoietic tissue
5
Effector T cells Mø LPS specific T cells. Thus the overriding goal is to be able to manipu-
late the donor HSC inoculum in such a way as to avoid GvHD
but to still be able to mediate a GvT effect. 6
GVHD tissue damage TNFα Clinical Aspects of aGvHD
lymphoid, skin, gut, liver Target cell apoptosis induction Usually developing within the first 3 months after transplantation,
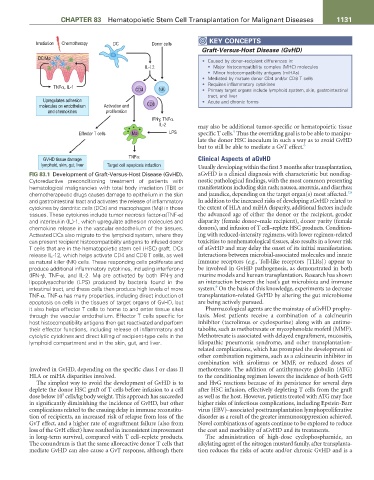
FIG 83.1 Development of Graft-Versus-Host Disease (GvHD). aGvHD is a clinical diagnosis with characteristic but nondiag-
Cytoreductive preconditioning treatment of patients with nostic pathological findings, with the most common presenting
hematological malignancies with total body irradiation (TBI) or manifestations including skin rash; nausea, anorexia, and diarrhea;
7,8
chemotherapeutic drugs causes damage to epithelium in the skin and jaundice, depending on the target organ(s) most affected.
and gastrointestinal tract and activates the release of inflammatory In addition to the increased risks of developing aGvHD related to
cytokines by dendritic cells (DCs) and macrophages (Mϕ) in those the extent of HLA and miHA disparity, additional factors include
tissues. These cytokines include tumor necrosis factor-α(TNF-α) the advanced age of either the donor or the recipient, gender
and interleukin (IL)-1, which upregulate adhesion molecules and disparity (female donor–male recipient), donor parity (female
chemokine release in the vascular endothelium of the tissues. donors), and infusion of T cell–replete HSC products. Condition-
Activated DCs also migrate to the lymphoid system, where they ing with reduced-intensity regimens, with lower regimen-related
can present recipient histocompatibility antigens to infused donor toxicities to nonhematological tissues, also results in a lower risk
T cells that are in the hematopoietic stem cell (HSC) graft. DCs of aGvHD and may delay the onset of its initial manifestation.
release IL-12, which helps activate CD4 and CD8 T cells, as well Interactions between microbial-associated molecules and innate
as natural killer (NK) cells. These responding cells proliferate and immune receptors (e.g., Toll-like receptors [TLRs]) appear to
produce additional inflammatory cytokines, including interferon-γ be involved in GvHD pathogenesis, as demonstrated in both
(IFN-γ), TNF-α, and IL-2. Mϕ are activated by both IFN-γ and murine models and human transplantation. Research has shown
lipopolysaccharide (LPS) produced by bacteria found in the an interaction between the host’s gut microbiota and immune
9
intestinal tract, and these cells then produce high levels of more system. On the basis of this knowledge, experiments to decrease
TNF-α. TNF-α has many properties, including direct induction of transplantation-related GvHD by altering the gut microbiome
apoptosis on cells in the tissues of target organs of GvHD, but are being actively pursued.
it also helps effector T cells to home to and enter tissue sites Pharmacological agents are the mainstay of aGvHD prophy-
through the vascular endothelium. Effector T cells specific for laxis. Most patients receive a combination of a calcineurin
host histocompatibility antigens then get reactivated and perform inhibitor (tacrolimus or cyclosporine) along with an antime-
their effector functions, including release of inflammatory and tabolite, such as methotrexate or mycophenolate mofetil (MMF).
cytolytic cytokines and direct killing of recipient-type cells in the Methotrexate is associated with delayed engraftment, mucositis,
lymphoid compartment and in the skin, gut, and liver. idiopathic pneumonia syndrome, and other transplantation-
related complications, which has prompted the development of
other combination regimens, such as a calcineurin inhibitor in
combination with sirolimus or MMF, or reduced doses of
involved in GvHD, depending on the specific class I or class II methotrexate. The addition of antithymocyte globulin (ATG)
HLA or miHA disparities involved. to the conditioning regimen lowers the incidence of both GvH
The simplest way to avoid the development of GvHD is to and HvG reactions because of its persistence for several days
deplete the donor HSC graft of T cells before infusion to a cell after HSC infusion, effectively depleting T cells from the graft
5
dose below 10 cells/kg body weight. This approach has succeeded as well as the host. However, patients treated with ATG may face
in significantly diminishing the incidence of GvHD, but other higher risks of infectious complications, including Epstein-Barr
complications related to the ensuing delay in immune reconstitu- virus (EBV)–associated posttransplantation lymphoproliferative
tion of recipients, an increased risk of relapse from loss of the disorder as a result of the greater immunosuppression achieved.
GvT effect, and a higher rate of engraftment failure (also from Novel combinations of agents continue to be explored to reduce
loss of the GvH effect) have resulted in inconsistent improvement the cost and morbidity of aGvHD and its treatments.
in long-term survival, compared with T cell–replete products. The administration of high-dose cyclophosphamide, an
The conundrum is that the same alloreactive donor T cells that alkylating agent of the nitrogen mustard family, after transplanta-
mediate GvHD can also cause a GvT response, although there tion reduces the risks of acute and/or chronic GvHD and is a