Page 1166 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1166
1130 PART NINE Transplantation
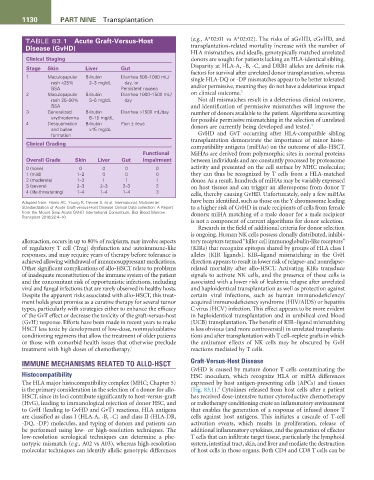
TABLE 83.1 Acute Graft-Versus-Host (e.g., A*02:01 vs A*02:02). The risks of aGvHD, cGvHD, and
Disease (GvHD) transplantation-related mortality increase with the number of
HLA mismatches, and ideally, genotypically matched unrelated
Clinical Staging donors are sought for patients lacking an HLA-identical sibling.
Stage Skin Liver Gut Disparity at HLA-A, -B, -C, and DRB1 alleles are definite risk
factors for survival after unrelated donor transplantation, whereas
1 Maculopapular Bilirubin Diarrhea 500–1000 mL/ single HLA-DQ or -DP mismatches appear to be better tolerated
rash <25% 2–3 mg/dL day, or
BSA Persistent nausea and/or permissive, meaning they do not have a deleterious impact
2
2 Maculopapular Bilirubin Diarrhea 1000–1500 mL/ on clinical outcome.
rash 25–50% 3–6 mg/dL day Not all mismatches result in a deleterious clinical outcome,
BSA and identification of permissive mismatches will improve the
3 Generalized Bilirubin Diarrhea >1500 mL/day number of donors available to the patient. Algorithms accounting
erythroderma 6–15 mg/dL for possible permissive mismatching in the selection of unrelated
4 Desquamation Bilirubin Pain ± ileus donors are currently being developed and tested. 3
and bullae >15 mg/dL
formation GvHD and GvT occurring after HLA-compatible sibling
transplantation demonstrate the importance of minor histo-
Clinical Grading
compatibility antigens (miHAs) on the outcome of allo-HSCT.
Functional MiHAs are derived from polymorphic sites in normal proteins
Overall Grade Skin Liver Gut Impairment between individuals and are constantly processed by proteasome
0 (none) 0 0 0 0 activity and presented on the cell surface by MHC molecules;
1 (mild) 1–2 0 0 0 they can thus be recognized by T cells from a HLA-matched
2 (moderate) 1–3 1 1 1 donor. As a result, hundreds of miHAs may be variably expressed
3 (severe) 2–3 2–3 2–3 2 on host tissues and can trigger an alloresponse from donor T
4 (life-threatening) 1–4 1–4 1–4 3 cells, thereby causing GvHD. Unfortunately, only a few miHAs
Adapted from: Harris AC, Young R, Devine S, et al. International, Multicenter have been identified, such as those on the Y chromosome leading
Standardization of Acute Graft-versus-Host Disease Clinical Data collection: A Report to a higher risk of GvHD in male recipients of cells from female
from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow donors; miHA matching of a male donor for a male recipient
Transplant 2016;22:4–10.
is not a component of current algorithms for donor selection.
Research in the field of additional criteria for donor selection
is ongoing. Human NK cells possess clonally distributed, inhibi-
alloreaction, occurs in up to 80% of recipients, may involve aspects tory receptors termed “killer cell immunoglobulin-like receptors”
of regulatory T cell (Treg) dysfunction and autoimmune-like (KIRs) that recognize epitopes shared by groups of HLA class I
responses, and may require years of therapy before tolerance is alleles (KIR ligands). KIR–ligand mismatching in the GvH
achieved allowing withdrawal of immunosuppressant medications. direction appears to result in lower risk of relapse- and nonrelapse-
Other significant complications of allo-HSCT relate to problems related mortality after allo-HSCT. Activating KIRs transduce
of inadequate reconstitution of the immune system of the patient signals to activate NK cells, and the presence of these cells is
and the concomitant risk of opportunistic infections, including associated with a lower risk of leukemia relapse after unrelated
viral and fungal infections that are rarely observed in healthy hosts. and haploidentical transplantation as well as protection against
Despite the apparent risks associated with allo-HSCT, this treat- certain viral infections, such as human immunodeficiency/
ment holds great promise as a curative therapy for several tumor acquired immunodeficiency syndrome (HIV/AIDS) or hepatitis
types, particularly with strategies either to enhance the efficacy C virus (HCV) infection. This effect appears to be more evident
of the GvT effect or decrease the toxicity of the graft-versus-host in haploidentical transplantation and in umbilical cord blood
(GvH) response. Efforts have been made in recent years to make (UCB) transplantation. The benefit of KIR–ligand mismatching
HSCT less toxic by development of low-dose, nonmyeloablative is less obvious (and more controversial) in unrelated transplanta-
conditioning regimens that allow the treatment of older patients tions and after transplantation with T cell–replete grafts in which
or those with comorbid health issues that otherwise preclude the antitumor effects of NK cells may be obscured by GvH
treatment with high doses of chemotherapy. 1 reactions mediated by T cells.
IMMUNE MECHANISMS RELATED TO ALLO-HSCT Graft-Versus-Host Disease
GvHD is caused by mature donor T cells contaminating the
Histocompatibility HSC inoculum, which recognize HLA or miHA differences
The HLA major histocompatibility complex (MHC; Chapter 5) expressed by host antigen-presenting cells (APCs) and tissues
4
is the primary consideration in the selection of a donor for allo- (Fig. 83.1). Cytokines released from host cells after a patient
HSCT, since its loci contribute significantly to host-versus-graft has received dose-intensive tumor cytoreductive chemotherapy
(HvG), leading to immunological rejection of donor HSC, and or radiotherapy conditioning create an inflammatory environment
to GvH (leading to GvHD and GvT) reactions. HLA antigens that enables the generation of a response of infused donor T
are classified as class I (HLA-A, -B, -C) and class II (HLA-DR, cells against host antigens. This initiates a cascade of T-cell
-DQ, -DP) molecules, and typing of donors and patients can activation events, which results in proliferation, release of
be performed using low- or high-resolution techniques. The additional inflammatory cytokines, and the generation of effector
low-resolution serological techniques can determine a phe- T cells that can infiltrate target tissue, particularly the lymphoid
notypic mismatch (e.g., A02 vs A03), whereas high-resolution system, intestinal tract, skin, and liver and mediate the destruction
molecular techniques can identify allelic genotypic differences of host cells in those organs. Both CD4 and CD8 T cells can be