Page 1180 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1180
CHaPTEr 84 Immunoglobulin Therapy: Replacement and Immunomodulation 1145
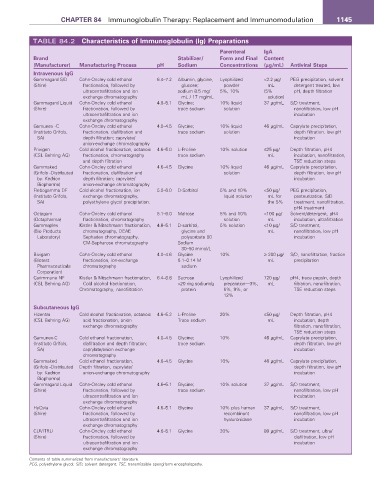
TABLE 84.2 Characteristics of Immunoglobulin (Ig) Preparations
Parenteral Iga
Brand Stabilizer/ Form and Final Content
(Manufacturer) Manufacturing Process pH Sodium Concentrations (µg/mL) antiviral Steps
Intravenous IgG
Gammagard S/D Cohn-Oncley cold ethanol 6.4–7.2 Albumin, glycine, Lyophilized <2.2 µg/ PEG precipitation, solvent
(Shire) fractionation, followed by glucose; powder mL detergent treated, low
ultracentrafiltration and ion sodium 8.5 mg/ 5%, 10% (5% pH, depth filtration
exchange chromatography mL / 17 mg/mL solution)
Gammagard Liquid Cohn-Oncley cold ethanol 4.6–5.1 Glycine; 10% liquid 37 µg/mL S/D treatment,
(Shire) fractionation, followed by trace sodium solution nanofiltration, low pH
ultracentrafiltration and ion incubation
exchange chromatography
Gamunex -C Cohn-Oncley cold ethanol 4.0–4.5 Glycine; 10% liquid 46 µg/mL Caprylate precipitation,
(Instituto Grifols, fractionation, diafiltration and trace sodium solution depth filtration, low pH
SA) depth filtration; caprylate/ incubation
anion-exchange chromatography
Privigen Cold alcohol fractionation, octanoic 4.6–5.0 L-Proline 10% solution ≤25 µg/ Depth filtration, pH4
(CSL Behring AG) fractionation, chromatography trace sodium mL incubation, nanofiltration,
and depth filtration TSE reduction steps
Gammaked Cohn-Oncley cold ethanol 4.6–4.5 Glycine 10% liquid 46 µg/mL Caprylate precipitation,
(Grifols -Distributed fractionation, diafiltration and solution depth filtration, low pH
by: Kedrion depth filtration; caprylate/ incubation
Biopharma) anion-exchange chromatography
Flebogamma DF Cold alcohol fractionation, ion 5.0–6.0 D-Sorbitol 5% and 10% <50 µg/ PEG precipitation,
(Instituto Grifols, exchange chromatography, liquid solution mL for pasteurization, S/D
SA) polyethylene glycol precipitation, the 5% treatment, nanofiltration,
pH4 treatment
Octagam Cohn-Oncley cold ethanol 5.1–6.0 Maltose 5% and 10% <100 µg/ Solvent/detergent, pH4
(Octapharma) fractionation, chromatography solution mL incubation, ultrafiltration
Gammaplex Kistler & Nitschmann fractionation, 4.8–5.1 D-sorbitol, 5% solution <10 µg/ S/D treatment,
(Bio Products chromatography, DEAE glycine and mL nanofiltration, low pH
Laboratory) Sephadex chromatography, polysorbate 80 incubation
CM-Sepharose chromatography Sodium
30–50 mmol/L
Bivigam Cohn-Oncley cold ethanol 4.0–4.6 Glycine 10% ≥ 200 µg/ S/D, nanofiltration, fraction
(Biotest fractionation, ion-exchange 0.1–0.14 M mL precipitation
Pharmaceuticals chromatography sodium
Corporation)
Carimmune NF Kistler & Nitschmann fractionation, 6.4–6.8 Sucrose Lyophilized 720 µg/ pH4, trace pepsin, depth
(CSL Behring AG) Cold alcohol fractionation, <20 mg sodium/g preparation—3%, mL filtration, nanofiltration,
Chromatography, nanofiltration protein 6%, 9%, or TSE reduction steps
12%
Subcutaneous IgG
Hizentra Cold alcohol fractionation, octanoic 4.6–5.2 L-Proline 20% ≤50 µg/ Depth filtration, pH4
(CSL Behring AG) acid fractionation, anion Trace sodium mL incubation, depth
exchange chromatography filtration, nanofiltration,
TSE reduction steps
Gamunex-C Cold ethanol fractionation, 4.0–4.5 Glycine; 10% 46 µg/mL Caprylate precipitation,
(Instituto Grifols, diafiltration and depth filtration; trace sodium depth filtration, low pH
SA) caprylate/anion exchange incubation
chromatography
Gammaked Cold ethanol fractionation, 4.6–4.5 Glycine 10% 46 µg/mL Caprylate precipitation,
(Grifols -Distributed Depth filtration, caprylate/ depth filtration, low pH
by: Kedrion anion-exchange chromatography incubation
Biopharma)
Gammagard Liquid Cohn-Oncley cold ethanol 4.6–5.1 Glycine; 10% solution 37 µg/mL S/D treatment,
(Shire) fractionation, followed by trace sodium nanofiltration, low pH
ultracentrafiltration and ion incubation
exchange chromatography
HyQvia Cohn-Oncley cold ethanol 4.6–5.1 Glycine 10% plus human 37 µg/mL S/D treatment,
(Shire) fractionation, followed by recombinant nanofiltration, low pH
ultracentrafiltration and ion hyaluronidase incubation
exchange chromatography
CUVITRU Cohn-Oncley cold ethanol 4.6–5.1 Glycine 20% 80 µg/mL S/D treatment, ultra/
(Shire) fractionation, followed by diafiltration, low pH
ultracentrafiltration and ion incubation
exchange chromatography
Contents of table summarized from manufacturers’ literature.
PEG, polyethylene glycol; S/D, solvent detergent; TSE, transmissible spongiform encephalopathy.