Page 357 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 357
CHaPTEr 23 Mast Cells, Basophils, and Mastocytosis 337
carboxypeptidase A3 (CPA3). Accordingly, the anatomically
distinct mouse mast cell subpopulations express very different
profiles of proteases (and correspondingly different GAG composi-
tions), dependent on the microenvironment. 16
In contrast to the mouse, human mast cells express only a
single mast cell–specific chymase gene and thus exhibit less
heterogeneity compared with their rodent counterparts. Human
mast cells can express up to four tryptase genes (α, β, γ and δ),
and CPA3. All human mast cells express tryptase β, and some
also express chymase, CPA3, and cathepsin G. Human mast cells
expressing tryptase in the absence (MC T ) or presence (MC TC )
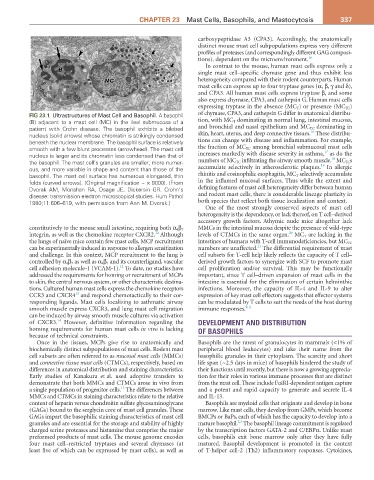
FIG 23.1 Ultrastructures of Mast Cell and Basophil. A basophil of chymase, CPA3, and cathepsin G differ in anatomical distribu-
(B) adjacent to a mast cell (MC) in the ileal submucosa of a tion, with MC T dominating in normal lung, intestinal mucosa,
patient with Crohn disease. The basophil exhibits a bilobed and bronchial and nasal epithelium and MC TC dominating in
16
nucleus (solid arrows) whose chromatin is strikingly condensed skin, heart, uterus, and deep connective tissues. These distribu-
beneath the nuclear membrane. The basophil surface is relatively tions can change with disease and inflammation. For example,
smooth with a few blunt processes (arrowhead). The mast cell the fraction of MC TC among bronchial submucosal mast cells
17
nucleus is larger and its chromatin less condensed than that of increases markedly with disease severity in asthma, as do the
18
the basophil. The mast cell’s granules are smaller, more numer- numbers of MC TC infiltrating the airway smooth muscle. MC TC s
19
ous, and more variable in shape and content than those of the accumulate selectively in atherosclerotic plaques. In allergic
basophil. The mast cell surface has numerous elongated, thin rhinitis and eosinophilic esophagitis, MC T selectively accumulate
folds (curved arrows). (Original magnification ~ × 9000). (From in the inflamed mucosal surfaces. Thus while the extent and
Dvorak AM, Monahan RA, Osage JE, Dickersin GR. Crohn’s defining features of mast cell heterogeneity differ between human
disease: transmission electron microscopical studies. Hum Pathol and rodent mast cells, there is considerable lineage plasticity in
1980;11:606–619, with permission from Ann M. Dvorak.) both species that reflect both tissue localization and context.
One of the most strongly conserved aspects of mast cell
heterogeneity is the dependency, or lack thereof, on T cell–derived
accessory growth factors. Athymic nude mice altogether lack
constitutively to the mouse small intestine, requiring both α 4 β 7 MMCs in the intestinal mucosa despite the presence of wild-type
20
12
integrin, as well as the chemokine receptor CXCR2. Although levels of CTMCs in the same organ. MC T are lacking in the
the lungs of naïve mice contain few mast cells, MCP recruitment intestines of humans with T-cell immunodeficiencies, but MC TC
21
can be experimentally induced in response to allergen sensitization numbers are unaffected. The differential requirement of mast
and challenge. In this context, MCP recruitment to the lung is cell subsets for T-cell help likely reflects the capacity of T cell–
controlled by α 4 β 7 as well as α 4 β 1 and its counterligand, vascular derived growth factors to synergize with SCF to promote mast
12
cell adhesion molecule-1 (VCAM-1). To date, no studies have cell proliferation and/or survival. This may be functionally
addressed the requirements for homing or recruitment of MCPs important, since T cell–driven expansion of mast cells in the
to skin, the central nervous system, or other characteristic destina- intestine is essential for the elimination of certain helminthic
tions. Cultured human mast cells express the chemokine receptors infections. Moreover, the capacity of IL-4 and IL-9 to alter
13
CCR3 and CXCR4 and respond chemotactically to their cor- expression of key mast cell effectors suggests that effector systems
responding ligands. Mast cells localizing to asthmatic airway can be modulated by T cells to suit the needs of the host during
smooth muscle express CXCR3, and lung mast cell migration immune responses. 9,11
can be induced by airway smooth muscle cultures via activation
14
of CXCR3. However, definitive information regarding the DEVELOPMENT AND DISTRIBUTION
homing requirements for human mast cells in vivo is lacking OF BASOPHILS
because of technical constraints.
Once in the tissues, MCPs give rise to anatomically and Basophils are the rarest of granulocytes in mammals (<1% of
biochemically distinct subpopulations of mast cells. Rodent mast peripheral blood leukocytes) and take their name from the
cell subsets are often referred to as mucosal mast cells (MMCs) basophilic granules in their cytoplasm. The scarcity and short
and connective tissue mast cells (CTMCs), respectively, based on life span (~2.5 days in mice) of basophils hindered the study of
differences in anatomical distribution and staining characteristics. their functions until recently, but there is now a growing apprecia-
Early studies of Kanakura et al. used adoptive transfers to tion for their roles in various immune processes that are distinct
demonstrate that both MMCs and CTMCs arose in vivo from from the mast cell. These include FcεRI-dependent antigen capture
15
a single population of progenitor cells. The differences between and a potent and rapid capacity to generate and secrete IL-4
MMCs and CTMCs in staining characteristics relate to the relative and IL-13.
content of heparin versus chondroitin sulfate glycosaminoglycans Basophils are myeloid cells that originate and develop in bone
(GAGs) bound to the serglycin core of mast cell granules. These marrow. Like mast cells, they develop from GMPs, which become
GAGs impart the basophilic staining characteristics of mast cell BMCPs or BaPs, each of which has the capacity to develop into a
2,3
granules and are essential for the storage and stability of highly mature basophil. The basophil lineage commitment is regulated
charged serine proteases and histamine that comprise the major by the transcription factors GATA-2 and C/EBPα. Unlike mast
preformed products of mast cells. The mouse genome encodes cells, basophils exit bone marrow only after they have fully
four mast cell–restricted tryptases and several chymases (at matured. Basophil development is promoted in the context
least five of which can be expressed by mast cells), as well as of T-helper cell-2 (Th2) inflammatory responses. Cytokines,