Page 442 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 442
422 ParT ThrEE Host Defenses to Infectious Agents
AIDS, who are highly susceptible to C. neoformans infection, p30 Chronic granulomatous disease (CGD) is a well-known
expression is decreased on NK cells leading to defective perforin example of a genetic disorder associated with increased susceptibil-
release and reduced microbicidal activity. IL-12 restored p30 ity to invasive bacterial and fungal infections, especially invasive
expression and fungal killing in a mouse model of C. neoformans aspergillosis. CGD is caused by mutations in genes encoding
infection by NK cells. These data indicate a direct role for NK subunits of the NADPH complex (e.g., CYBA, CYBB NCF1, NCF2,
cells against Cryptococcus through direct recognition by a cell NCF4), which results in defective ROS production, a critical step
surface receptor. Evidence for the role of NK cells against invasive in the microbial killing process. 34,35 Recent work has shown that
fungal pathogens has been expanded to show that they play a deficiencies in ROS production resulting from a mutation in a
nonredundant role in clearing C. glabrata infections. The human subunit of the NADPH complex also result in defective autophagy.
NK receptor p46 and its mouse orthologue natural cytotoxic- These defects prevent autophagy-dependent inhibition of IL-1β
ity triggering receptor 1 (NCR1) bind C. glabrata. In vitro, NK and increased inflammasome activation. Blocking of IL-1 protects
cells lacking NCR1 show reduced capacity to kill C. glabrata. mice with CGD from invasive aspergillosis and colitis and restores
−/−
Indeed, NCR1 mice have increased susceptibility to C. glabrata autophagic function in monocytes derived from patients with
infection. The ligands for p46/NCR1 are discrete members of CGD. 36
a family of proteins called EPA (epithelial adhesion), which are Chronic mucocutaneous candidiasis (CMC) is an immune
glycan-binding lectins and permit attachment of fungal cells to disorder characterized by persistent or recurrent candidal infec-
host cells. tions of the skin, nails, and mucous membranes. Primarily, CMC
appears to be associated with defects in IL-17 signaling. A direct
GENETIC SUSCEPTIBILITIES TO INVASIVE role for IL-17 signaling in the protection against CMC was
FUNGAL INFECTIONS demonstrated in two families with deficiencies in the IL-17
37
receptor A (IL-17RA) and the cytokine IL-17F. As mentioned
Although certain clinical diagnoses and medical interventions previously, mutations in dectin-1 cause increased susceptibility
are associated with susceptibility to fungal infections, not all to CMC as a result of impaired IL-1β production and a reduced
38
patients in these high-risk groups become infected. Moreover, frequency of Th17 cells. Similarly, patients with CARD9 deficien-
individuals lacking traditional risk factors (e.g., immunosup- cies have increased susceptibility to invasive fungal infections
pression, HIV, etc.) can develop chronic or invasive fungal resulting from decreased Th17 cells, reduced chemokine/cytokine
infections, suggesting that additional factors confer susceptibility. production, and impaired neutrophil killing. 39
Primary immunodeficiencies (PIDs) include a group of hereditary CMC is also common in patients with autoimmune polyen-
immune disorders that render patients more susceptible to docrinopathy syndrome type 1 (APS-1), an autosomal recessive
infection. Recent studies of PIDs have provided the opportunity disorder caused by defects in the autoimmune regulator (AIRE)
to define some of the genetic and molecular mechanisms that gene. In these patients, CMC results from the generation of
40
contribute to infection. A list of genes associated with susceptibility autoantibodies against IL-17 and IL-22. Hyper-IgE syndrome
to fungal infection are summarized in Table 29.1. (HIES) is an autosomal dominant or recessive immune disorder
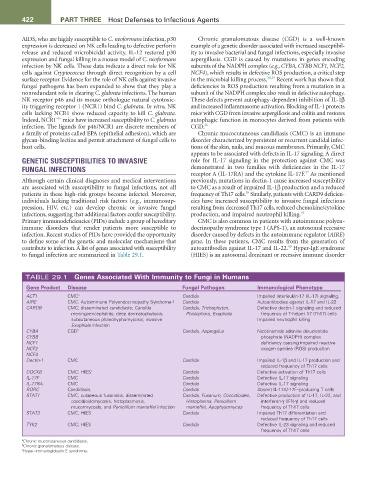
TABLE 29.1 Genes associated With Immunity to Fungi in humans
Gene Product Disease Fungal Pathogen Immunological Phenotype
ACT1 CMC a Candida Impaired interleukin-17 (IL-17) signaling.
AIRE CMC; Autoimmune Polyendocrinopathy Syndrome-1 Candida Autoantibodies against IL-17 and IL-22
CARD9 CMC; disseminated candidiasis; Candida Candida, Trichophyton, Defective dectin-1 signaling and reduced
meningoencephalitis; deep dermatophytosis; Phialophora, Exophiala frequency of T-helper 17 (Th17) cells
subcutaneous phaeohyphomycosis; invasive Impaired neutrophil killing
Exophiala infection
CYBA CGD b Candida, Aspergillus Nicotinamide adenine dinucleotide
CYBB phosphate (NADPH) complex
NCF1 deficiency causing impaired reactive
NCF2 oxygen species (ROS) production
NCF4
Dectin-1 CMC Candida Impaired IL-1β and IL-17 production and
reduced frequency of Th17 cells
DOCK8 CMC; HIES c Candida Defective activation of Th17 cells
IL-17F CMC Candida Defective IL-17 signaling
IL-17RA CMC Candida Defective IL-17 signaling
RORC Candidiasis Candida Absent IL-17A/-17F–producing T cells
STAT1 CMC, cutaneous fusariosis, disseminated Candida, Fusarium, Coccidioides, Defective production of IL-17, IL-22, and
coccidioidomycosis, histoplasmosis, Histoplasma, Penicillium interferon-γ (IFN-γ) and reduced
mucormycosis, and Penicillium marneffei infection marneffei, Apophysomyces frequency of Th17 cells
STAT3 CMC; HIES Candida Impaired Th17 differentiation and
reduced frequency of Th17 cells
TYK2 CMC; HIES Candida Defective IL-23 signaling and reduced
frequency of Th17 cells
a Chronic mucocutaneous candidiasis.
b Chronic granulomatous disease.
c Hyper–immunoglobulin E syndrome.