Page 652 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 652
626 ParT FIVE Allergic Diseases
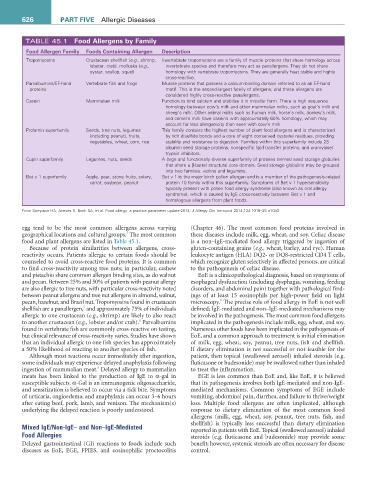
TABLE 45.1 Food allergens by Family
Food allergen Family Foods Containing allergen Description
Tropomyosins Crustacean shellfish (e.g., shrimp, Invertebrate tropomyosins are a family of muscle proteins that share homology across
lobster, crab), mollusks (e.g., invertebrate species and therefore may act as panallergens. They do not share
oyster, scallop, squid) homology with vertebrate tropomyosins. They are generally heat stable and highly
cross-reactive.
Parvalbumins/EF-hand Vertebrate fish and frogs Muscle proteins that possess a calcium-binding domain referred to as an EF-hand
proteins motif. This is the second-largest family of allergens, and these allergens are
considered highly cross-reactive panallergens.
Casein Mammalian milk Function to bind calcium and stabilize it in micellar form. There is high sequence
homology between cow’s milk and other mammalian milks, such as goat’s milk and
sheep’s milk. Other animal milks such as human milk, horse’s milk, donkey’s milk,
and camel’s milk have caseins with approximately 60% homology, which may
account for less allergenicity than seen with cow’s milk.
Prolamin superfamily Seeds, tree nuts, legumes This family contains the highest number of plant food allergens and is characterized
(including peanut), fruits, by rich disulfide bonds and a core of eight conserved cysteine residues, providing
vegetables, wheat, corn, rice stability and resistance to digestion. Families within this superfamily include 2S
albumin seed storage proteins, nonspecific lipid transfer proteins, and α-amylase/
trypsin inhibitors.
Cupin superfamily Legumes, nuts, seeds A large and functionally diverse superfamily of proteins termed seed storage globulins
that share a β-barrel structural core domain. Seed storage globulins may be grouped
into two families: vicilins and legumins.
Bet v 1 superfamily Apple, pear, stone fruits, celery, Bet v 1 is the major birch pollen allergen and is a member of the pathogenesis-related
carrot, soybean, peanut protein 10 family within this superfamily. Symptoms of Bet v 1 hypersensitivity
typically present with pollen food allergy syndrome (also known as oral allergy
syndrome), which is caused by IgE cross-reactivity between Bet v 1 and
homologous allergens from plant foods.
From Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014;134:1016–25 e1043.
egg tend to be the most common allergens across varying (Chapter 46). The most common food proteins involved in
7
geographical locations and cultural groups. The most common these diseases include milk, egg, wheat, and soy. Celiac disease
food and plant allergens are listed in Table 45.1. is a non–IgE-mediated food allergy triggered by ingestion of
Because of protein similarities between allergens, cross- gluten-containing grains (e.g., wheat, barley, and rye). Human
reactivity occurs. Patients allergic to certain foods should be leukocyte antigen (HLA) DQ2- or DQ8-restricted CD4 T cells,
counseled to avoid cross-reactive food proteins. It is common which recognize gluten selectively in affected persons, are critical
to find cross-reactivity among tree nuts; in particular, cashew to the pathogenesis of celiac disease.
and pistachio share common allergen binding sites, as do walnut EoE is a clinicopathological diagnosis, based on symptoms of
and pecan. Between 25% and 50% of patients with peanut allergy esophageal dysfunction (including dysphagia, vomiting, feeding
are also allergic to tree nuts, with particular cross-reactivity noted disorders, and abdominal pain) together with pathological find-
between peanut allergens and tree nut allergens in almond, walnut, ings of at least 15 eosinophils per high-power field on light
9
pecan, hazelnut, and Brazil nut. Tropomyosins found in crustacean microscopy. The precise role of food allergy in EoE is not well
5
shellfish are a panallergen, and approximately 75% of individuals defined; IgE-mediated and non–IgE-mediated mechanisms may
allergic to one crustacean (e.g., shrimp) are likely to also react be involved in the pathogenesis. The most common food allergens
8
to another crustacean (e.g., lobster and/or crab). Parvalbumins implicated in the pathogenesis include milk, egg, wheat, and soy.
found in vertebrate fish are commonly cross-reactive on testing, Numerous other foods have been implicated in the pathogenesis of
but clinical relevance of cross-reactivity varies. Studies have shown EoE, and a common approach to treatment is initial elimination
that an individual allergic to one fish species has approximately of milk, egg, wheat, soy, peanut, tree nuts, fish and shellfish.
a 50% likelihood of reacting to another species of fish. If dietary elimination is not successful or not feasible for the
Although most reactions occur immediately after ingestion, patient, then topical (swallowed aerosol) inhaled steroids (e.g.
some individuals may experience delayed anaphylaxis following fluticasone or budesonide) may be swallowed rather than inhaled
5
ingestion of mammalian meat. Delayed allergy to mammalian to treat the inflammation.
meats has been linked to the production of IgE to α-gal in EGE is less common than EoE and, like EoE, it is believed
susceptible subjects. α-Gal is an immunogenic oligosaccharide, that its pathogenesis involves both IgE-mediated and non-IgE-
and sensitization is believed to occur via a tick bite. Symptoms mediated mechanisms. Common symptoms of EGE include
of urticaria, angioedema, and anaphylaxis can occur 3–6 hours vomiting, abdominal pain, diarrhea, and failure to thrive/weight
after eating beef, pork, lamb, and venison. The mechanism(s) loss. Multiple food allergens are often implicated, although
underlying the delayed reaction is poorly understood. response to dietary elimination of the most common food
allergens (milk, egg, wheat, soy, peanut, tree nuts, fish, and
Mixed IgE/Non-IgE– and Non–IgE-Mediated shellfish) is typically less successful than dietary elimination
reported in patients with EoE. Topical (swallowed aerosol) inhaled
Food Allergies steroids (e.g. fluticasone and budesonide) may provide some
Delayed gastrointestinal (GI) reactions to foods include such benefit; however, systemic steroids are often necessary for disease
diseases as EoE, EGE, FPIES, and eosinophilic proctocolitis control.