Page 840 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 840
812 Part SIX Systemic Immune Diseases
$257$ $257,& %5$1&+(6 Analysis of clonal CD4 populations has yielded identical T-cell
receptors (TCRs) in independent temporal artery biopsies from
the same patient, strongly supportive for antigen driving T-cell
20
behavior in the tissue microenvironment. Wall-embedded
vasDCs serve as antigen-presenting cells (APCs); the nature of
the antigen remains unknown. Reports about infectious agents
isolated from temporal arteries have notoriously not been
confirmed in independent studies. The older adult host is expected
to accumulate viral and bacterial microorganisms, and locally
deposited pathogens may not indicate causal involvement but,
rather, represent the vascular microbiota. Carefully designed
experiments need to probe for a specific role of pathogens isolated
from temporal artery tissue.
T helper 1 (Th1) cells are a key pathogenic element in GCA.
Interferon-γ (IFN-γ), the Th1 marker cytokine, has multiple
disease-relevant functions: activating endothelial cells and
macrophages, regulating wall remodeling, inducing microvascular
14
neoangiogenesis, and driving intimal-layer expansion. IFN-γ
production is predominantly produced in the adventitia, where
T cells are instructed by vasDCs. Th1 cells are present in lesions
from untreated patients and persist despite prolonged cortico-
21
steroid therapy, suggesting independence of vasculitogenic
Th1 cells from the steroid-sensitive acute-phase response and
$GYHQWLWDO WKLFNHQLQJ $GYHQWLWDO QHRDQJLRJHQHVLV
emphasizing their role as stabilizers of chronic-persistent lesions.
0HGLDO WKLQQLQJ ZDOO GHVWUXFWLRQ 0\RILEUREODVW PRELOL]DWLRQ During early vasculitis, Th1 cells are accompanied by Th17 cells.
$QHXU\VP IRUPDWLRQ DQG SUROLIHUDWLRQ However, Th17 cells are highly sensitive to steroid therapy and
21
'LVVHFWLRQ ,QWLPDO K\SHUSODVLD chronic lesions are Th17 depleted. Comparative analysis of
/XPLQDO RFFOXVLRQ
sequential temporal artery biopsy tissues harvested from patients
before and after treatment has demonstrated that adaptive
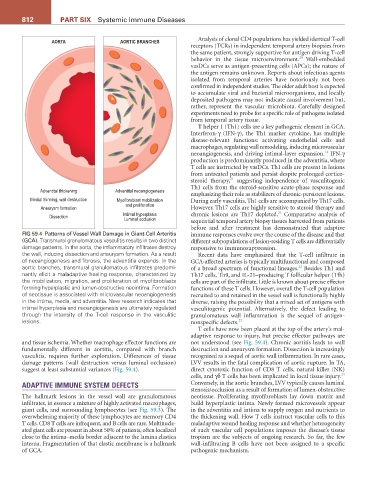
FIG 59.4 Patterns of Vessel Wall Damage in Giant-Cell Arteritis immune responses evolve over the course of the disease and that
(GCA). Transmural granulomatous vasculitis results in two distinct different subpopulations of lesion-residing T cells are differentially
damage patterns. In the aorta, the inflammatory infiltrates destroy responsive to immunosuppression.
the wall, inducing dissection and aneurysm formation. As a result Recent data have emphasized that the T-cell infiltrate in
of neoangiogenesis and fibrosis, the adventitia expands. In the GCA-affected arteries is typically multifunctional and composed
aortic branches, transmural granulomatous infiltrates predomi- of a broad spectrum of functional lineages. Besides Th1 and
22
nantly elicit a maladaptive healing response, characterized by Th17 cells, Th9, and IL-21–producing T follicular helper (Tfh)
the mobilization, migration, and proliferation of myofibroblasts cells are part of the infiltrate. Little is known about precise effector
forming hyperplastic and lumen-obstructive neointima. Formation functions of these T cells. However, overall the T-cell population
of neotissue is associated with microvascular neoangiogenesis recruited to and retained in the vessel wall is functionally highly
in the intima, media, and adventitia. New research indicates that diverse, raising the possibility that a mixed set of antigens with
intimal hyperplasia and neoangiogenesis are ultimately regulated vasculitogenic potential. Alternatively, the defect leading to
through the intensity of the T-cell response in the vasculitic granulomatous wall inflammation is the sequel of antigen-
lesions. nonspecific defects. 22
T cells have now been placed at the top of the artery’s mal-
adaptive response to injury, but precise effector pathways are
and tissue ischemia. Whether macrophage effector functions are not understood (see Fig. 59.4). Chronic aortitis leads to wall
fundamentally different in aortitis, compared with branch destruction and aneurysm formation. Dissection is increasingly
vasculitis, requires further exploration. Differences of tissue recognized as a sequel of aortic wall inflammation. In rare cases,
damage patterns (wall destruction versus luminal occlusion) LVV results in the fatal complication of aortic rupture. In TA,
suggest at least substantial variances (Fig. 59.4). direct cytotoxic function of CD8 T cells, natural killer (NK)
23
cells, and γδ T cells has been implicated in local tissue injury.
ADAPTIVE IMMUNE SYSTEM DEFECTS Conversely, in the aortic branches, LVV typically causes luminal
stenosis/occlusion as a result of formation of lumen-obstructive
The hallmark lesions in the vessel wall are granulomatous neotissue. Proliferating myofibroblasts lay down matrix and
infiltrates, in essence a mixture of highly activated macrophages, build hyperplastic intima. Newly formed microvessels appear
giant cells, and surrounding lymphocytes (see Fig. 59.3). The in the adventitia and intima to supply oxygen and nutrients to
overwhelming majority of these lymphocytes are memory CD4 the thickening wall. How T cells instruct vascular cells to this
T cells. CD8 T cells are infrequent, and B cells are rare. Multinucle- maladaptive wound healing response and whether heterogeneity
ated giant cells are present in about 50% of patients, often localized of such vascular cell populations imposes the disease’s tissue
close to the intima–media border adjacent to the lamina elastica tropism are the subjects of ongoing research. So far, the few
interna. Fragmentation of that elastic membrane is a hallmark wall-infiltrating B cells have not been assigned to a specific
of GCA. pathogenic mechanism.