Page 983 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 983
CHaPTEr 70 Autoimmune Thyroid Diseases 949
N
LRRs
α/A subunit
Cleaved
region (residues
~316–366)
FIG 70.2 Diffuse lymphocyte infiltrate and thyrocyte hyperplasia
in a patient with Graves disease.
activation increases. T cells are exposed to thyroid antigens,
resulting in an autoimmune response at the time of immune Plasma membrane
13
reconstitution. A similar phenomenon has been seen in indi
viduals with multiple sclerosis (MS) treated with alemtuzumab, β/B subunit
the lymphocytedepleting antiCD52 antibody. An alternative
explanation is the weakening of physiological antiinflammatory
pathways, which unleashes the immune system. As more novel
biological agents become available, this phenomenon is likely
to become more common.
C
KEY CONCEPTS
Environmental Factors Known to Influence TMD
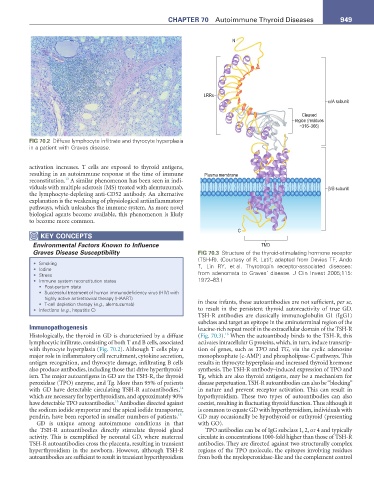
Graves Disease Susceptibility FIG 70.3 Structure of the thyroid-stimulating hormone receptor
(TSH-R). (Courtesy of R. Latif; adapted from Davies TF, Ando
• Smoking T, Lin RY, et al. Thyrotropin receptor-associated diseases:
• Iodine
• Stress from adenomata to Graves’ disease. J Clin Invest 2005;115:
• Immune system reconstitution states 1972–83.)
• Post-partum state
• Successful treatment of human immunodeficiency virus (HIV) with
highly active antiretroviral therapy (HAART)
• T-cell depletion therapy (e.g., alemtuzumab) in these infants, these autoantibodies are not sufficient, per se,
• Infections (e.g., hepatitis C) to result in the persistent thyroid autoreactivity of true GD.
TSHR antibodies are classically immunoglobulin G1 (IgG1)
subclass and target an epitope in the aminoterminal region of the
Immunopathogenesis leucinerich repeat motif in the extracellular domain of the TSHR
16
Histologically, the thyroid in GD is characterized by a diffuse (Fig. 70.3). When the autoantibody binds to the TSHR, this
lymphocytic infiltrate, consisting of both T and B cells, associated activates intracellular G proteins, which, in turn, induce transcrip
with thyrocyte hyperplasia (Fig. 70.2). Although T cells play a tion of genes, such as TPO and TG, via the cyclic adenosine
major role in inflammatory cell recruitment, cytokine secretion, monophosphate (cAMP) and phospholipaseC pathways. This
antigen recognition, and thyrocyte damage, infiltrating B cells results in thyrocyte hyperplasia and increased thyroid hormone
also produce antibodies, including those that drive hyperthyroid synthesis. The TSHR antibody–induced expression of TPO and
ism. The major autoantigens in GD are the TSHR, the thyroid Tg, which are also thyroid antigens, may be a mechanism for
peroxidase (TPO) enzyme, and Tg. More than 95% of patients disease perpetuation. TSHR autoantibodies can also be “blocking”
14
with GD have detectable circulating TSHR autoantibodies, in nature and prevent receptor activation. This can result in
which are necessary for hyperthyroidism, and approximately 90% hypothyroidism. These two types of autoantibodies can also
15
have detectable TPO autoantibodies. Antibodies directed against coexist, resulting in fluctuating thyroid function. Thus although it
the sodium iodide symporter and the apical iodide transporter, is common to equate GD with hyperthyroidism, individuals with
pendrin, have been reported in smaller numbers of patients. 15 GD may occasionally be hypothyroid or euthyroid (presenting
GD is unique among autoimmune conditions in that with GO).
the TSHR autoantibodies directly stimulate thyroid gland TPO antibodies can be of IgG subclass 1, 2, or 4 and typically
activity. This is exemplified by neonatal GD, where maternal circulate in concentrations 1000fold higher than those of TSHR
TSHR autoantibodies cross the placenta, resulting in transient antibodies. They are directed against two structurally complex
hyperthyroidism in the newborn. However, although TSHR regions of the TPO molecule, the epitopes involving residues
autoantibodies are sufficient to result in transient hyperthyroidism from both the myeloperoxidaselike and the complement control