Page 1738 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 1738
CHAPTER 124: Toxicology in Adults 1207
38,000 of these cases were treated in health care facilities; 25,764 patients (SI Units)
received N-acetylcysteine (NAC); 1193 had major complications; M per L g per mL
235 died. In late 2010, the FDA approved an IV formulation of
1
acetaminophen. 6000 1000
The evolution of acetaminophen toxicity is often divided into four 5000
phases. Phase I refers to the first 24 hours after ingestion. During this 4000
100
period, acetaminophen is absorbed and metabolized, glutathione stores 500
are depleted, and hepatotoxicity begins. Patients are alert but may expe- 3000
rience nausea, vomiting, anorexia, malaise, pallor, or diaphoresis toward
the end of this phase. These nonspecific signs and symptoms create the 2000
potential for a missed diagnosis. Furthermore, when acetaminophen is 1300 200
combined with another drug such as codeine or oxycodone, the effects
of the other drug may mask the initial subtle manifestations of acet- 1000 150
aminophen poisoning. 900
Phase II occurs 24 to 72 hours after untreated ingestion. During 800
this period, patients may develop right upper quadrant pain and 700 100
minor abnormalities of liver function consisting of elevation of hepatic 600
enzymes, prothrombin time, and bilirubin. Phase III (72-96 hours after 500 Probable hepatic
untreated ingestion) is characterized by continued hepatic necrosis, toxicity
hepatic encephalopathy, disseminated intravascular coagulation, and 400
jaundice. Liver function abnormalities typically peak during phase III. 50
It is not unusual for serum aspartate aminotransferase levels to rise to 300 Possible hepatic
10,000 IU/L or higher. Extreme elevation of alanine aminotransferase 250 toxicity
may also be seen. Rare phase III sequelae include hemorrhagic pancre-
atitis, myocardial necrosis, and acute renal failure. Phase IV takes place Acetaminophen plasma concentration 200
4 days to 2 weeks after ingestion. During this period, the patient may
die, fully recover without chronic liver disease, or require emergent liver
transplantation. Some patients with untreated acetaminophen poisoning
exhibit a pattern of rising prothrombin time, ammonia, and bilirubin, as 100
levels of hepatic enzymes decline, indicating fulminant hepatic necrosis. 90 No hepatic
toxicity
A product of normal acetaminophen metabolism is responsible for 80
hepatotoxicity. As acetaminophen is metabolized by the cytochrome 70
P-450 mixed oxidase system, a toxic product (N-acetyl-p-benzoquinone 60 10
imine or NAPQI) is rapidly detoxified by hepatic intracellular 50
glutathione. In overdose cases, glutathione is depleted, allowing the
101
toxic metabolite to combine with hepatic cell proteins and cause cen- 40
trilobular necrosis with periportal sparing. Local production of NAPQI 5 25%
makes the liver the primary target, but other organs can be affected. 30
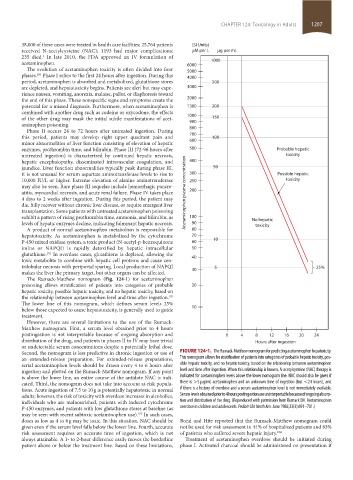
The Rumack-Matthew nomogram (Fig. 124-1) for acetaminophen
poisoning allows stratification of patients into categories of probable 20
hepatic toxicity, possible hepatic toxicity, and no hepatic toxicity, based on
the relationship between acetaminophen level and time after ingestion.
102
The lower line of this nomogram, which defines serum levels 25%
below those expected to cause hepatotoxicity, is generally used to guide 10
treatment.
However, there are several limitations to the use of the Rumack-
Matthew nomogram. First, a serum level obtained prior to 4 hours
postingestion is not interpretable because of ongoing absorption and 0 4 8 12 16 20 24
distribution of the drug, and patients in phases II to IV may have trivial Hours after ingestion
or undetectable serum concentrations despite a potentially lethal dose.
Second, the nomogram is less predictive in chronic ingestion or use of FIGURE 124-1. The Rumack-Matthew nomogram for predicting acetaminophen hepatoxicity.
an extended-release preparation. For extended-release preparations, This nomogram allows for stratification of patients into categories of probable hepatic toxicity, pos-
serial acetaminophen levels should be drawn every 4 to 6 hours after sible hepatic toxicity, and no hepatic toxicity, based on the relationship between acetaminophen
ingestion and plotted on the Rumack-Matthew nomogram. If any point level and time after ingestion. When this relationship is known, N-acetylcysteine (NAC) therapy is
is above the lower line, an entire course of the antidote NAC is indi- indicated for acetaminophen levels above the lower nomogram line. NAC should also be given if
cated. Third, the nomogram does not take into account at-risk popula- there is >5 µg/mL acetaminophen and an unknown time of ingestion (but <24 hours), and
tions. Acute ingestion of 7.5 to 10 g is potentially hepatotoxic in normal if there is a history of overdose and a serum acetaminophen level is not immediately available.
adults; however, the risk of toxicity with overdose increases in alcoholics, Serum levels obtained prior to 4 hours postingestion are uninterpretable because of ongoing absorp-
individuals who are malnourished, patients with induced cytochrome tion and distribution of the drug. (Reproduced with permission from Rumack BH. Acetaminophen
P-450 enzymes, and patients with low glutathione stores at baseline (as overdose in children and adolescents. Pediatr Clin North Am. June 1986;33(3):691–701.)
may be seen with recent subtoxic acetaminophen use). In such cases,
103
doses as low as 4 to 6 g may be toxic. In this situation, NAC should be Bond and Hite reported that the Rumack-Matthew nomogram could
given even if the serum level falls below the lower line. Fourth, accurate not be used for risk assessment in 44% of hospitalized patients and 83%
risk assessment requires an accurate time of ingestion, which is not of patients who suffered severe hepatic injury. 104
always attainable. A 1- to 2-hour difference easily moves the borderline Treatment of acetaminophen overdose should be initiated during
patient above or below the treatment line. Based on these limitations, phase I. Activated charcoal should be administered on presentation if
section11.indd 1207 1/19/2015 10:51:59 AM