Page 452 - Clinical Hematology_ Theory _ Procedures ( PDFDrive )
P. 452
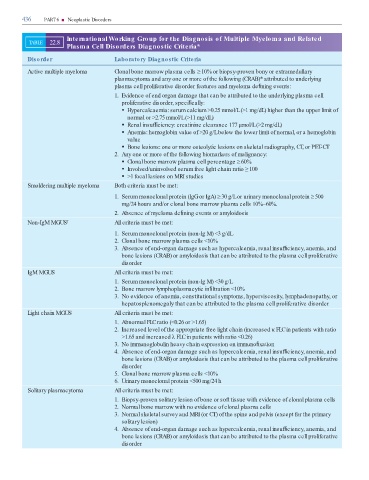
436 PART 6 ■ Neoplastic Disorders
International Working Group for the Diagnosis of Multiple Myeloma and Related
TABLE 22.8
Plasma Cell Disorders Diagnostic Criteria*
Disorder Laboratory Diagnostic Criteria
Active multiple myeloma Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary
plasmacytoma and any one or more of the following (CRAB)* attributed to underlying
plasma cell proliferative disorder features and myeloma de ning events:
1. Evidence of end organ damage that can be attributed to the underlying plasma cell
proliferative disorder, speci cally:
Hypercalcaemia: serum calcium >0.25 mmol/L (>1 mg/dL) higher than the upper limit of
normal or >2.75 mmol/L (>11 mg/dL)
Renal insuf ciency: creatinine clearance 177 µmol/L (>2 mg/dL)
Anemia: hemoglobin value of >20 g/L below the lower limit of normal, or a hemoglobin
value
Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or PET-CT
2. Any one or more of the following biomarkers of malignancy:
Clonal bone marrow plasma cell percentage ≥60%
Involved/uninvolved serum free light chain ratio ≥100
>1 focal lesions on MRI studies
Smoldering multiple myeloma Both criteria must be met:
1. Serum monoclonal protein (IgG or IgA) ≥30 g/L or urinary monoclonal protein ≥500
mg/24 hours and/or clonal bone marrow plasma cells 10%–60%.
2. Absence of myeloma de ning events or amyloidosis
Non-IgM MGUS † All criteria must be met:
1. Serum monoclonal protein (non-Ig M) <3 g/dL
2. Clonal bone marrow plasma cells <10%
3. Absence of end-organ damage such as hypercalcemia, renal insuf ciency, anemia, and
bone lesions (CRAB) or amyloidosis that can be attributed to the plasma cell proliferative
disorder
IgM MGUS All criteria must be met:
1. Serum monoclonal protein (non-Ig M) <30 g/L
2. Bone marrow lymphoplasmacytic in ltration <10%
3. No evidence of anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or
hepatosplenomegaly that can be attributed to the plasma cell proliferative disorder
Light chain MGUS All criteria must be met:
1. Abnormal FLC ratio (<0.26 or >1.65)
2. Increased level of the appropriate free light chain (increased κ FLC in patients with ratio
>1.65 and increased λ FLC in patients with ratio <0.26)
3. No immunoglobulin heavy chain expression on immuno xation
4. Absence of end-organ damage such as hypercalcemia, renal insuf ciency, anemia, and
bone lesions (CRAB) or amyloidosis that can be attributed to the plasma cell proliferative
disorder
5. Clonal bone marrow plasma cells <10%
6. Urinary monoclonal protein <500 mg/24 h
Solitary plasmacytoma All criteria must be met:
1. Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells
2. Normal bone marrow with no evidence of clonal plasma cells
3. Normal skeletal survey and MRI (or CT) of the spine and pelvis (except for the primary
solitary lesion)
4. Absence of end-organ damage such as hypercalcemia, renal insuf ciency, anemia, and
bone lesions (CRAB) or amyloidosis that can be attributed to the plasma cell proliferative
disorder