Page 287 - 9780077418427.pdf
P. 287
/Users/user-f465/Desktop
tiL12214_ch10_251-274.indd Page 264 9/3/10 6:11 PM user-f465
tiL12214_ch10_251-274.indd Page 264 9/3/10 6:11 PM user-f465 /Users/user-f465/Desktop
products had a total mass of 36 u. Again, this is in accord with
the law of conservation of mass.
The equation says that 4 u of hydrogen will combine with
32 u of oxygen. Thus, hydrogen and oxygen combine in a mass
ratio of 4:32, which reduces to 1:8. So 1 g of hydrogen will com- Hydrogen + Chlorine Hydrogen chloride Hydrogen chloride
bine with 8 g of oxygen, and, in fact, they will combine in this
ratio no matter what the measurement units are (gram, kilo-
gram, pound, etc.). They always combine in this mass ratio
because this is the mass of the individual reactants. A
Back in the early 1800s, John Dalton (1766–1844) attempted
to work out a table of atomic weights as he developed his atomic
theory. Dalton made two major errors in determining the atomic
weights, including (1) measurement errors about mass ratios of
combining elements and (2) incorrect assumptions about the
formula of the resulting compound. For water, for example, Hydrogen Hydrogen + Oxygen Water vapor Water vapor
Dalton incorrectly measured that 5.5 g of oxygen combined
with 1.0 g of hydrogen. He assumed that one atom of hydrogen
combined with one atom of oxygen, resulting in a formula of
HO. Thus, Dalton concluded that the atomic mass of oxygen B
was 5.5 u, and the atomic mass of hydrogen was 1.0 u. Incor- FIGURE 10.14 Reacting gases combine in ratios of small,
rect atomic weights for hydrogen and oxygen led to conflicting whole number volumes when the temperature and pressure are the
formulas for other substances, and no one could show that the same for each volume. (A) One volume of hydrogen gas combines
atomic theory worked. with one volume of chlorine gas to yield two volumes of hydrogen
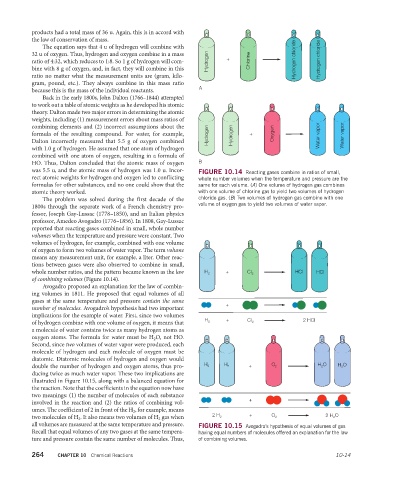
The problem was solved during the first decade of the chloride gas. (B) Two volumes of hydrogen gas combine with one
1800s through the separate work of a French chemistry pro- volume of oxygen gas to yield two volumes of water vapor.
fessor, Joseph Gay-Lussac (1778–1850), and an Italian physics
professor, Amedeo Avogadro (1776–1856). In 1808, Gay-Lussac
reported that reacting gases combined in small, whole number
volumes when the temperature and pressure were constant. Two
volumes of hydrogen, for example, combined with one volume
of oxygen to form two volumes of water vapor. The term volume
means any measurement unit, for example, a liter. Other reac-
tions between gases were also observed to combine in small,
whole number ratios, and the pattern became known as the law H 2 + CI 2 HCI HCI
of combining volumes (Figure 10.14).
Avogadro proposed an explanation for the law of combin-
ing volumes in 1811. He proposed that equal volumes of all
gases at the same temperature and pressure contain the same +
number of molecules. Avogadro’s hypothesis had two important
implications for the example of water. First, since two volumes
of hydrogen combine with one volume of oxygen, it means that H 2 + CI 2 2 HCI
a molecule of water contains twice as many hydrogen atoms as
oxygen atoms. The formula for water must be H 2 O, not HO.
Second, since two volumes of water vapor were produced, each
molecule of hydrogen and each molecule of oxygen must be
diatomic. Diatomic molecules of hydrogen and oxygen would
double the number of hydrogen and oxygen atoms, thus pro- H 2 H 2 + O 2 H 2 O H 2 O
ducing twice as much water vapor. These two implications are
illustrated in Figure 10.15, along with a balanced equation for
the reaction. Note that the coefficients in the equation now have
two meanings: (1) the number of molecules of each substance +
involved in the reaction and (2) the ratios of combining vol-
umes. The coefficient of 2 in front of the H 2 , for example, means
two molecules of H 2 . It also means two volumes of H 2 gas when 2 H 2 + O 2 2 H 2 O
all volumes are measured at the same temperature and pressure. FIGURE 10.15 Avogadro’s hypothesis of equal volumes of gas
Recall that equal volumes of any two gases at the same tempera- having equal numbers of molecules offered an explanation for the law
ture and pressure contain the same number of molecules. Thus, of combining volumes.
264 CHAPTER 10 Chemical Reactions 10-14