Page 16 - PRE-U STPM CHEMISTRY TERM 1
P. 16
Chemistry Term 1 STPM
15
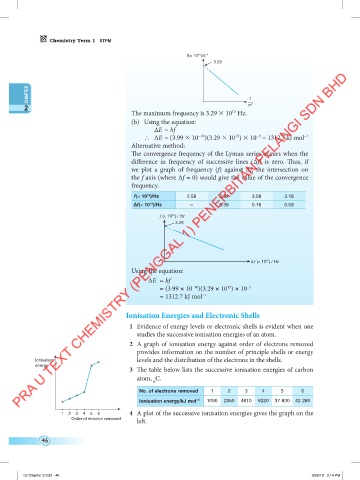
f(× 10 )/s –1
3.29
CHAPTER 1
2 n 2
The maximum frequency is 3.29 10 Hz.
15
(b) Using the equation:
∆E = hf
15
–3
∴ ∆E = (3.99 10 )(3.29 10 ) 10 = 1312.7 kJ mol –1
–10
Alternative method:
The convergence frequency of the Lyman series occurs when the
difference in frequency of successive lines (∆f) is zero. Thus, if
we plot a graph of frequency (f) against ∆f, the intersection on
the f axis (where ∆f = 0) would give the value of the convergence
frequency.
f(× 10 )/Hz 2.56 2.92 3.08 3.16
15
∆f(× 10 )/Hz – 0.36 0.16 0.08
15
15
ƒ (× 10 ) / Hz
3.29
Δƒ (× 10 ) / Hz
15
Using the equation:
∆E = hf
= (3.99 × 10 )(3.29 × 10 ) × 10 –3
–10
15
= 1312.7 kJ mol –1
Ionisation Energies and Electronic Shells
1 Evidence of energy levels or electronic shells is evident when one
studies the successive ionisation energies of an atom.
2 A graph of ionisation energy against order of electrons removed
provides information on the number of principle shells or energy
levels and the distribution of the electrons in the shells.
Ionisation
energy
3 The table below lists the successive ionisation energies of carbon
atom, C.
6
No. of electrons removed 1 2 3 4 5 6
Ionisation energy/kJ mol –1 1090 2350 4610 6220 37 830 42 280
4 A plot of the successive ionisation energies gives the graph on the
1 2 3 4 5 6
left.
Order of electron removed
46
02 Chapter 2.indd 46 3/26/18 3:14 PM