Page 40 - Ranger SPM 2022 - Science
P. 40
Science SPM Chapter 6 Electrochemistry
6. Which of the following is not an B Extraction of metals from mineral
HOTS
HOTS application of electrolysis in our daily ores
life? C Purification of metals
A Generation of electricity D Prevents rusting of iron
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
Subjective Questions
Section A (b) Based on the observations in
Table 1, state one inference.
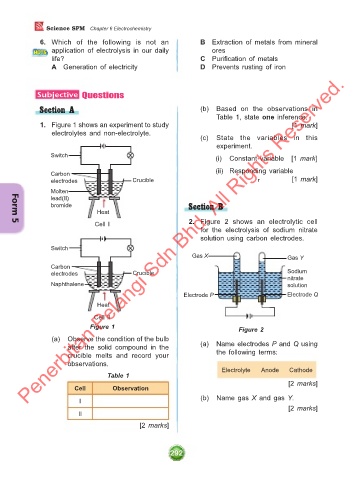
1. Figure 1 shows an experiment to study [1 mark]
electrolytes and non-electrolyte.
(c) State the variables in this
experiment.
Switch Switch (i) Constant variable [1 mark]
(ii) Responding variable
Carbon Carbon
electrodes Crucible electrodes Crucible [1 mark]
Molten Naphthalene
lead(II)
bromide Section B
Heat Heat
Cell I 2. Figure 2 shows an electrolytic cell
Form 5
for the electrolysis of sodium nitrate
solution using carbon electrodes.
Switch Switch
Gas X Gas Y
Carbon Carbon
electrodes Crucible electrodes Crucible Sodium
nitrate
Molten Naphthalene solution
lead(II) Electrode Q
bromide Electrode P
Heat Heat
Cell II
Figure 1 Figure 2
(a) Observe the condition of the bulb
after the solid compound in the (a) Name electrodes P and Q using
crucible melts and record your the following terms:
observations.
Electrolyte Anode Cathode
Table 1
[2 marks]
Cell Observation
I (b) Name gas X and gas Y.
[2 marks]
II
[2 marks]
292
F5 Chapter 6.indd 292 3/21/22 3:59 PM