Page 37 - Ranger SPM 2022 - Science
P. 37
Science SPM Chapter 6 Electrochemistry
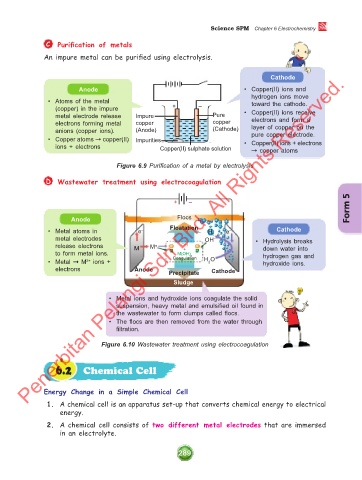
C Purification of metals
An impure metal can be purified using electrolysis.
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
Cathode
Anode • Copper(II) ions and
hydrogen ions move
• Atoms of the metal toward the cathode.
(copper) in the impure + – • Copper(II) ions receive
metal electrode release Impure Pure electrons and form a
electrons forming metal copper copper
anions (copper ions). (Anode) (Cathode) layer of copper on the
pure copper electrode.
• Copper atoms ➞ copper(II) Impurities • Copper(II) ions + electrons
ions + electrons Copper(II) sulphate solution ➞ copper atoms
Figure 6.9 Purification of a metal by electrolysis
D Wastewater treatment using electrocoagulation
Form 5
+ –
Anode Flocs
• Metal atoms in e – Floatation H 2 e – Cathode
metal electrodes OH – • Hydrolysis breaks
release electrons M M n OH down water into
+
to form metal ions. M(OH) n hydrogen gas and
• Metal ➞ M ions + Coagulation H O hydroxide ions.
2+
2
electrons Anode Precipitate Cathode
Sludge
• Metal ions and hydroxide ions coagulate the solid
suspension, heavy metal and emulsified oil found in
the wastewater to form clumps called flocs.
• The flocs are then removed from the water through
filtration.
Figure 6.10 Wastewater treatment using electrocoagulation
6.2 Chemical Cell
Energy Change in a Simple Chemical Cell
1. A chemical cell is an apparatus set-up that converts chemical energy to electrical
energy.
2. A chemical cell consists of two different metal electrodes that are immersed
in an electrolyte.
289
F5 Chapter 6.indd 289 3/21/22 3:59 PM