Page 36 - Ranger SPM 2022 - Science
P. 36
Science SPM Chapter 6 Electrochemistry
Applications of Electrolysis in Industries
A Extraction of metals
1. Metals such as potassium, sodium, calcium, magnesium and aluminium are more
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
reactive than carbon, hence these metals cannot be extracted by heating with
carbon.
2. Metals such as potassium, sodium, calcium, magnesium and aluminium must be
extracted using electrolysis.
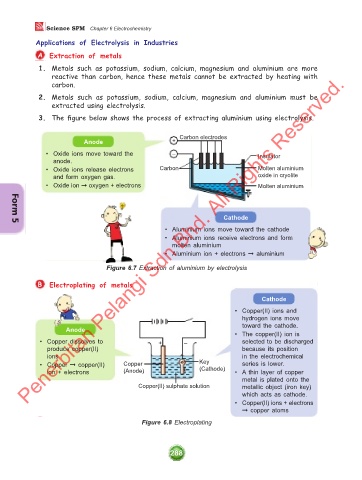
3. The figure below shows the process of extracting aluminium using electrolysis.
Anode + Carbon electrodes
• Oxide ions move toward the – Insulator
anode.
• Oxide ions release electrons Carbon Molten aluminium
and form oxygen gas. oxide in cryolite
• Oxide ion ➞ oxygen + electrons Molten aluminium
Cathode
Form 5
• Aluminium ions move toward the cathode
• Aluminium ions receive electrons and form
molten aluminium
• Aluminium ion + electrons ➞ aluminium
Figure 6.7 Extraction of aluminium by electrolysis
B Electroplating of metals
Cathode
• Copper(II) ions and
hydrogen ions move
toward the cathode.
Anode
• The copper(II) ion is
• Copper dissolves to + – selected to be discharged
produce copper(II) because its position
ions in the electrochemical
• Copper ➞ copper(II) Copper Key series is lower.
ion + electrons (Anode) (Cathode) • A thin layer of copper
metal is plated onto the
Copper(II) sulphate solution metallic object (iron key)
which acts as cathode.
• Copper(II) ions + electrons
➞ copper atoms
Figure 6.8 Electroplating
288
F5 Chapter 6.indd 288 3/21/22 3:59 PM