Page 38 - Ranger SPM 2022 - Science
P. 38
Science SPM Chapter 6 Electrochemistry
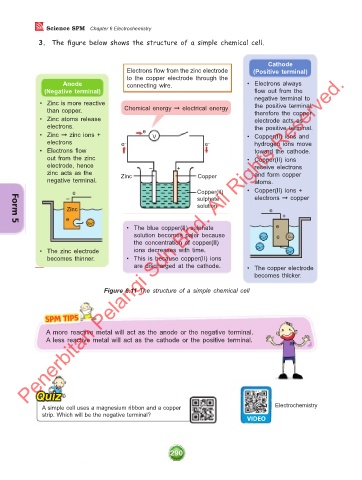
3. The figure below shows the structure of a simple chemical cell.
Cathode
Electrons flow from the zinc electrode (Positive terminal)
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
to the copper electrode through the
Anode connecting wire. • Electrons always
(Negative terminal) flow out from the
negative terminal to
• Zinc is more reactive the positive terminal,
than copper. Chemical energy ➞ electrical energy therefore the copper
• Zinc atoms release electrode acts as
electrons. the positive terminal.
• Zinc ➞ zinc ions + e – V • Copper(II) ions and
electrons e – e – hydrogen ions move
• Electrons flow toward the cathode.
out from the zinc • Copper(II) ions
electrode, hence – + receive electrons
zinc acts as the Zinc Copper and form copper
negative terminal. atoms.
e Copper(II) • Copper(II) ions +
– sulphate electrons ➞ copper
solution
Zinc e
e +
Zn 2+
Form 5
• The blue copper(II) sulphate e
solution becomes paler because Cu 2+ e Cu
the concentration of copper(II)
• The zinc electrode ions decreases with time. Cu 2+ Cu 2+
becomes thinner. • This is because copper(II) ions
are discharged at the cathode. • The copper electrode
becomes thicker.
Figure 6.11 The structure of a simple chemical cell
SPM TIPS
A more reactive metal will act as the anode or the negative terminal.
A less reactive metal will act as the cathode or the positive terminal.
Quiz
A simple cell uses a magnesium ribbon and a copper Electrochemistry
strip. Which will be the negative terminal?
VIDEO
290
F5 Chapter 6.indd 290 3/21/22 3:59 PM