Page 7 - Focus SPM KSSM F4 2020 - Chemistry
P. 7
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
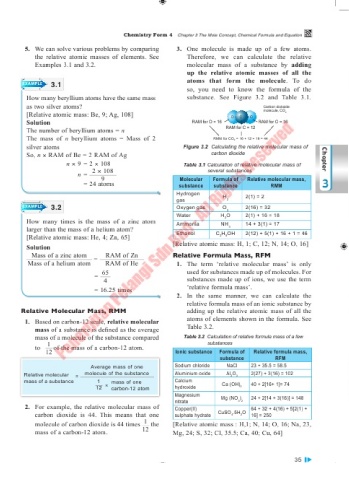
5. We can solve various problems by comparing 3. One molecule is made up of a few atoms.
the relative atomic masses of elements. See Therefore, we can calculate the relative
Examples 3.1 and 3.2. molecular mass of a substance by adding
up the relative atomic masses of all the
atoms that form the molecule. To do
EXAMPLE 3.1
so, you need to know the formula of the
How many beryllium atoms have the same mass substance. See Figure 3.2 and Table 3.1.
as two silver atoms? Carbon diokside
[Relative atomic mass: Be, 9; Ag, 108] O C O molecule, CO 2
Solution RAM for O = 16 RAM for O = 16
The number of beryllium atoms = n RAM for C = 12
The mass of n beryllium atoms = Mass of 2 RMM for CO 2 = 16 + 12 + 16 = 44
silver atoms Figure 3.2 Calculating the relative molecular mass of
So, n × RAM of Be = 2 RAM of Ag carbon dioxide
n × 9 = 2 × 108 Table 3.1 Calculation of relative molecular mass of Chapter
n = 2 × 108 Molecular several substances
9
Formula of
Relative molecular mass,
= 24 atoms substance substance RMM 3
Hydrogen H 2(1) = 2
gas 2
EXAMPLE 3.2 Oxygen gas O 2 2(16) = 32
Water H O 2(1) + 16 = 18
2
How many times is the mass of a zinc atom Ammonia NH 14 + 3(1) = 17
larger than the mass of a helium atom? 3
[Relative atomic mass: He, 4; Zn, 65] Ethanol C H OH 2(12) + 5(1) + 16 + 1 = 46
5
2
Solution [Relative atomic mass: H, 1; C, 12; N, 14; O, 16]
Mass of a zinc atom RAM of Zn Relative Formula Mass, RFM
Mass of a helium atom = RAM of He 1. The term ‘relative molecular mass’ is only
65 used for substances made up of molecules. For
=
4 substances made up of ions, we use the term
= 16.25 times ‘relative formula mass’.
2. In the same manner, we can calculate the
relative formula mass of an ionic substance by
Relative Molecular Mass, RMM adding up the relative atomic mass of all the
1. Based on carbon-12 scale, relative molecular atoms of elements shown in the formula. See
mass of a substance is defined as the average Table 3.2.
mass of a molecule of the substance compared Table 3.2 Calculation of relative formula mass of a few
substances
to 1 of the mass of a carbon-12 atom.
12 Ionic substance Formula of Relative formula mass,
substance RFM
Average mass of one Sodium chloride NaCl 23 + 35.5 = 58.5
Relative molecular = molecule of the substance Aluminium oxide Al O 3 2(27) + 3(16) = 102
2
mass of a substance 1 mass of one Calcium Ca (OH) 40 + 2[16+ 1]= 74
×
12
carbon-12 atom hydroxide 2
Magnesium Mg (NO ) 24 + 2[14 + 3(16)] = 148
nitrate 3 2
2. For example, the relative molecular mass of Copper(II) 64 + 32 + 4(16) + 5[2(1) +
carbon dioxide is 44. This means that one sulphate hydrate CuSO .5H O 16] = 250
4
2
molecule of carbon dioxide is 44 times 1 the [Relative atomic mass : H,1; N, 14; O, 16; Na, 23,
mass of a carbon-12 atom. 12 Mg, 24; S, 32; Cl, 35.5; Ca, 40; Cu, 64]
35
03 SPM CHEMISTRY F4.indd 35 27/02/2020 11:23 AM
Jisim molekul relatif bahan = Jisim purata satu molekul bahan
/ x jisim satu atom karbon-12
1
12