Page 28 - Ranger SPM 2022 Chemistry
P. 28
Chemistry SPM Chapter 2 Carbon Compound
• Fractional distillation is • Alcohol burns in excess
carried out to produce oxygen to produce carbon
ethanol with a higher purity. dioxide and water, burns
• Fermentation is very with a blue flame without
effective when carried out soot.
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
at an optimal temperature • C H OH(l) + 3O (g) →
2
2
5
of 37 °C because a higher 2CO (g) + 3H O(g)
2
2
temperature causes enzyme
to be denatured. (b) Oxidation
(b) Hydration • Alcohol undergoes oxidation
• Alcohol is produced through to produce ethanoic acid in
hydration by passing hot the presence of oxidising
steam over ethene. agents.
• The conditions needed for • Two commonly used oxidising
hydration: agents are:
(i) Temperature: 300 C (i) Acidified potassium
o
(ii) Pressure: 60 atm dichromate(VI) solution,
(iii) Catalyst: Phosphoric acid K Cr O .
2
2
7
• C H (g) + H O(g) (ii) Acidified potassium
2
4
2
H PO 4 manganate(VII) solution,
3
C H OH(l) KMnO .
300 °C, 60 atm 2 5 4
2. Alcohols undergo three chemical • C H OH(l) + 2[O] →
2
5
reactions: CH COOH(aq) + H O(l)
3
2
(a) Combustion
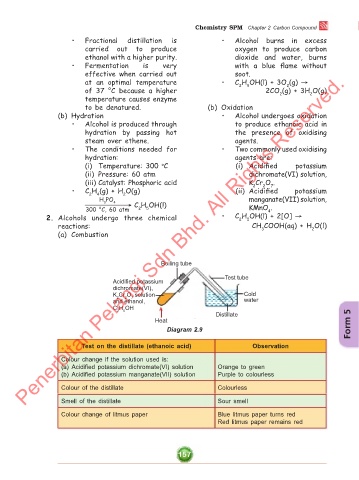
Boiling tube
Test tube
Acidified potassium
dichromate(VI),
K Cr O solution Cold
2
2
7
and ethanol, water
C H OH
2 5
Distillate
Heat Form 5
Diagram 2.9
Test on the distillate (ethanoic acid) Observation
Colour change if the solution used is:
(a) Acidified potassium dichromate(VI) solution Orange to green
(b) Acidified potassium manganate(VII) solution Purple to colourless
Colour of the distillate Colourless
Smell of the distillate Sour smell
Colour change of litmus paper Blue litmus paper turns red
Red litmus paper remains red
157
02.5 RANGER SPM CHEMISTRY 2P.indd 157 29/03/2022 2:28 PM