Page 31 - Ranger SPM 2022 Chemistry
P. 31
Chemistry SPM Chapter 2 Carbon Compound
• Magnesium powder reacts Ester
with ethanoic acid producing
magnesium ethanoate and 1. Ester is produced through the
hydrogen. esterification reaction between
• Mg(s) + 2CH COOH(aq) → alcohol and carboxylic acid.
the surface of water.Sdn Bhd. All Rights Reserved.
3
(CH COO) Mg(aq) + H (g) 2. The general formula of ester is
3
2
2
• Zinc reacts with ethanoic C H COOC H , m = 0, 1, 2 … and
2m+1
m
acid producing zinc ethanoate n = 1, 2, 3… n 2n+1
and hydrogen.
• Zn(s) + 2CH COOH(aq) → 3. The functional group of ester is
3
(CH COO) Zn(aq) + H (g) carboxylate group, —COO—.
3
2
2
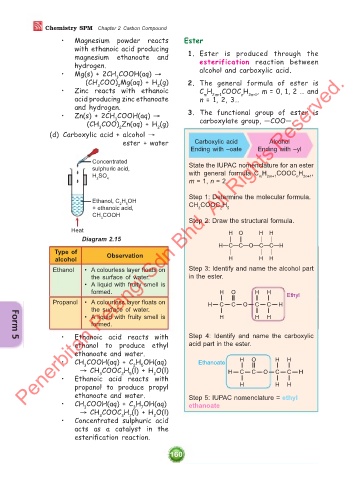
(d) Carboxylic acid + alcohol →
ester + water Carboxylic acid Alcohol
Ending with –oate Ending with –yl
Concentrated
sulphuric acid, State the IUPAC nomenclature for an ester
H SO with general formula C H 2m+1 COOC H 2n+1 ,
m
n
2 4
m = 1, n = 2.
Step 1: Determine the molecular formula.
Ethanol, C H OH
2
5
+ ethanoic acid, CH COOC H 5
2
3
CH COOH
3
Step 2: Draw the structural formula.
Heat H O H H
Diagram 2.15 & ' & &
H!C!C!O!C!C!H
Type of & & &
alcohol Observation H H H
Penerbitan Pelangi
Ethanol • A colourless layer floats on Step 3: Identify and name the alcohol part
in the ester.
• A liquid with fruity smell is
formed. H O H H Ethyl
Propanol • A colourless layer floats on H C C O C C H
the surface of water.
• A liquid with fruity smell is H H H
formed.
• Ethanoic acid reacts with Step 4: Identify and name the carboxylic
Form 5
ethanol to produce ethyl acid part in the ester.
ethanoate and water.
• CH COOH(aq) + C H OH(aq) Ethanoate H O H H
2
3
5
→ CH COOC H (l) + H O(l) H C C O C C H
2
2
5
3
• Ethanoic acid reacts with
propanol to produce propyl H H H
ethanoate and water. Step 5: IUPAC nomenclature = ethyl
• CH COOH(aq) + C H OH(aq) ethanoate
7
3
3
→ CH COOC H (l) + H O(l)
2
3
7
3
• Concentrated sulphuric acid
acts as a catalyst in the
esterification reaction.
160
02.5 RANGER SPM CHEMISTRY 2P.indd 160 29/03/2022 2:28 PM