Page 30 - Ranger SPM 2022 Chemistry
P. 30
Chemistry SPM Chapter 2 Carbon Compound
• Ethanoic acid reacts with • Ethanoic acid reacts with
copper(II) oxide forming sodium carbonate producing
copper(II) ethanoate and sodium ethanoate, carbon
water. dioxide and water.
• 2CH COOH(aq) + CuO(s) → • 2CH COOH(aq) + Na CO (s)
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
3
3
3
2
(CH COO) Cu (aq) + H O(l) → 2CH COONa(aq) + CO (g)
2
2
2
3
3
• Ethanoic acid reacts with + H O(l)
2
sodium hydroxide solution
producing sodium ethanoate (c) Carboxylic acid + metal →
and water. carboxylate salt + hydrogen
• CH COOH(aq) + NaOH(aq)
3
→ CH COONa(aq) + H O(l)
3
2
Lighted
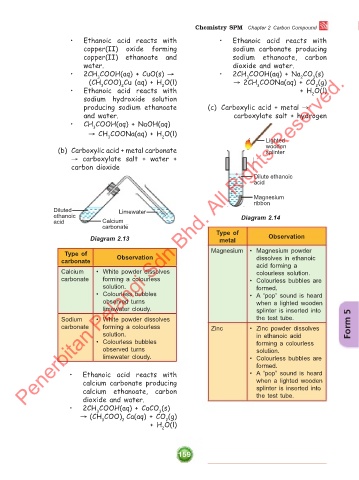
(b) Carboxylic acid + metal carbonate wooden
splinter
→ carboxylate salt + water +
carbon dioxide
Dilute ethanoic
acid
Magnesium
ribbon
Diluted Limewater
ethanoic Diagram 2.14
acid Calcium
carbonate
Type of
Diagram 2.13 metal Observation
Magnesium • Magnesium powder
Type of
carbonate Observation dissolves in ethanoic
acid forming a
Calcium • White powder dissolves colourless solution.
carbonate forming a colourless • Colourless bubbles are
solution. formed.
• Colourless bubbles • A “pop” sound is heard
observed turns when a lighted wooden
limewater cloudy. splinter is inserted into
Sodium • White powder dissolves the test tube.
carbonate forming a colourless Zinc • Zinc powder dissolves Form 5
solution. in ethanoic acid
• Colourless bubbles forming a colourless
observed turns solution.
limewater cloudy. • Colourless bubbles are
formed.
• Ethanoic acid reacts with • A “pop” sound is heard
calcium carbonate producing when a lighted wooden
calcium ethanoate, carbon splinter is inserted into
dioxide and water. the test tube.
• 2CH COOH(aq) + CaCO (s)
3
3
→ (CH COO) Ca(aq) + CO (g)
2
3
2
+ H O(l)
2
159
02.5 RANGER SPM CHEMISTRY 2P.indd 159 29/03/2022 2:28 PM