Page 26 - Ranger SPM 2022 Chemistry
P. 26
Chemistry SPM Chapter 2 Carbon Compound
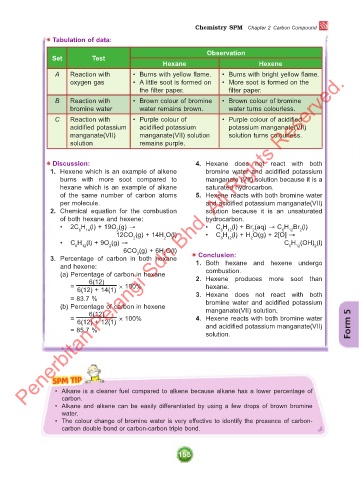
Tabulation of data:
Observation
Set Test
Hexane Hexene
= 85.7 %Pelangi Sdn Bhd. All Rights Reserved.
A Reaction with • Burns with yellow flame. • Burns with bright yellow flame.
oxygen gas • A little soot is formed on • More soot is formed on the
the filter paper. filter paper.
B Reaction with • Brown colour of bromine • Brown colour of bromine
bromine water water remains brown. water turns colourless.
C Reaction with • Purple colour of • Purple colour of acidified
acidified potassium acidified potassium potassium manganate(VII)
manganate(VII) manganate(VII) solution solution turns colourless.
solution remains purple.
Discussion: 4. Hexane does not react with both
1. Hexene which is an example of alkene bromine water and acidified potassium
burns with more soot compared to manganate (VII) solution because it is a
hexane which is an example of alkane saturated hydrocarbon.
of the same number of carbon atoms 5. Hexene reacts with both bromine water
per molecule. and acidified potassium manganate(VII)
2. Chemical equation for the combustion solution because it is an unsaturated
of both hexane and hexene: hydrocarbon.
• 2C H (l) + 19O (g) → • C H (l) + Br (aq) → C H Br (l)
6 14 2 6 12 2 6 12 2
12CO (g) + 14H O(l) • C H (l) + H O(g) + 2[O] →
2
2
12
6
2
• C H (l) + 9O (g) → C H (OH) (l)
6 12 2 6 12 2
6CO (g) + 6H O(l)
2
2
3. Percentage of carbon in both hexane Conclusion:
and hexene: 1. Both hexane and hexene undergo
(a) Percentage of carbon in hexane combustion.
6(12) 2. Hexene produces more soot than
= × 100% hexane.
6(12) + 14(1)
= 83.7 % 3. Hexane does not react with both
bromine water and acidified potassium
(b) Percentage of carbon in hexene manganate(VII) solution.
6(12) × 100% 4. Hexene reacts with both bromine water Form 5
=
Penerbitan
6(12) + 12(1)
and acidified potassium manganate(VII)
solution.
SPM TIP
• Alkane is a cleaner fuel compared to alkene because alkane has a lower percentage of
carbon.
• Alkane and alkene can be easily differentiated by using a few drops of brown bromine
water.
• The colour change of bromine water is very effective to identify the presence of carbon-
carbon double bond or carbon-carbon triple bond.
155
02.5 RANGER SPM CHEMISTRY 2P.indd 155 29/03/2022 2:28 PM