Page 145 - text book form physics kssm 2020
P. 145
Chapter 4
Heat
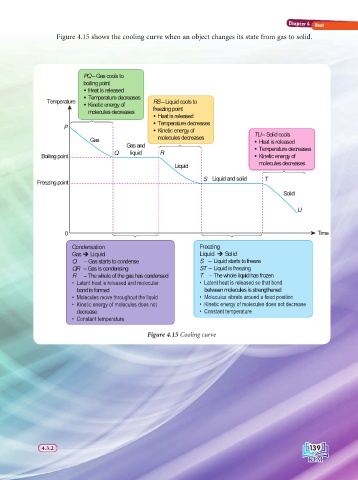
Figure 4.15 shows the cooling curve when an object changes its state from gas to solid.
PQ – Gas cools to
boiling point
Heat is released
Temperature decreases
Temperature Kinetic energy of RS – Liquid cools to
molecules decreases freezing point
64748 Heat is released
P Temperature decreases
Kinetic energy of TU – Solid cools
Gas molecules decreases Heat is released
Gas and 64748 Temperature decreases
Boiling point Q liquid R Kinetic energy of
Liquid molecules decreases
64748
S Liquid and solid T
Freezing point
Solid
U
0 64748 6447448 Time
Condensation Freezing
Gas Î Liquid Liquid Î Solid
Q – Gas starts to condense S – Liquid starts to freeze
QR – Gas is condensing ST – Liquid is freezing
R – The whole of the gas has condensed T – The whole liquid has frozen
/DWHQW KHDW LV UHOHDVHG DQG PROHFXODU /DWHQW KHDW LV UHOHDVHG VR WKDW ERQG
bond is formed between molecules is strengthened
0ROHFXOHV PRYH WKURXJKRXW WKH OLTXLG 0ROHFXOHV YLEUDWH DURXQG D IL[HG SRVLWLRQ
.LQHWLF HQHUJ\ RI PROHFXOHV GRHV QRW .LQHWLF HQHUJ\ RI PROHFXOHV GRHV QRW GHFUHDVH
decrease &RQVWDQW WHPSHUDWXUH
&RQVWDQW WHPSHUDWXUH
Figure 4.15 Cooling curve
139
139
139
4.3.2 139
4.3.2