Page 167 - text book form physics kssm 2020
P. 167
Chapter 4
Heat
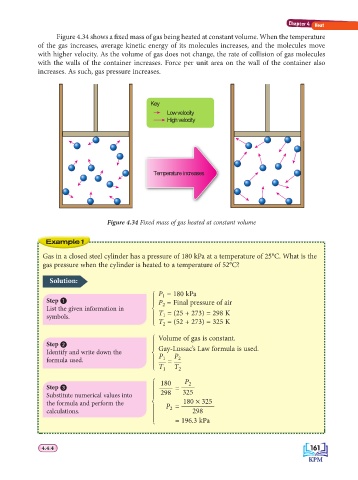
Figure 4.34 shows a fi xed mass of gas being heated at constant volume. When the temperature
of the gas increases, average kinetic energy of its molecules increases, and the molecules move
with higher velocity. As the volume of gas does not change, the rate of collision of gas molecules
with the walls of the container increases. Force per unit area on the wall of the container also
increases. As such, gas pressure increases.
Key
Low velocity
High velocity
Temperature increases
Figure 4.34 Fixed mass of gas heated at constant volume
Example 1
Gas in a closed steel cylinder has a pressure of 180 kPa at a temperature of 25°C. What is the
gas pressure when the cylinder is heated to a temperature of 52°C?
Solution:
14243
1
Step P = 180 kPa
P = Final pressure of air
2
List the given information in T = (25 + 273) = 298 K
1
symbols. T = (52 + 273) = 325 K
2
14243 P P
Step Volume of gas is constant.
Identify and write down the Gay-Lussac’s Law formula is used.
formula used. T 1 1 = T 2 2
14243 180 × 325
Step 180 = P 2
Substitute numerical values into 298 325
the formula and perform the P =
298
calculations. 2 = 196.3 kPa
161
161
4.4.4 161
4.4.4