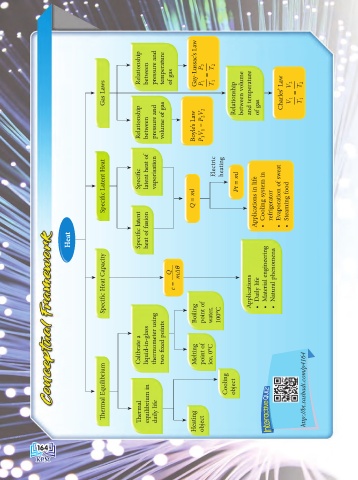
Page 170 - text book form physics kssm 2020
P. 170
Relationship between pressure and temperature of gas Gay-Lussac’s Law P 1 = P 2 T 2
Gas Laws T 1 Relationship between volume and temperature Charles’ Law V 1 = V 2 T 2 T 1
Relationship between pressure and volume of gas Boyle’s Law P 1 V 1 = P 2 V 2 of gas
c Latent Heat c Specifi latent heat of vaporisation Electric heating Pt = ml
Specifi Q = ml Applications in life Cooling system in refrigerator Evaporation of sweat • Steaming food
c latent heat of fusion • •
Heat Specifi
c Heat Capacity Q c = mΔθ • Material engineering • Natural phenomena
Specifi Boiling point of water, 100°C Applications • Daily life
Calibrate a liquid-in-glass thermometer using xed points two fi Melting point of ice, 0°C
ermal Equilibrium equilibrium in Cooling object http://bt.sasbadi.com/p4164
Th ermal Th daily life Heating object
164
164