Page 166 - text book form physics kssm 2020
P. 166
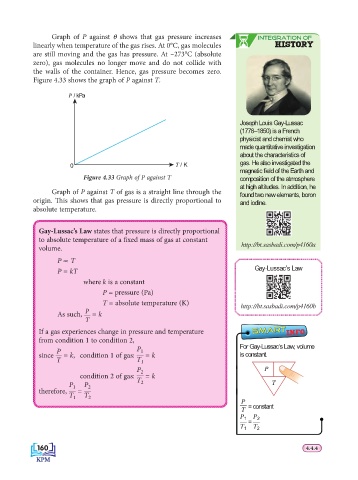
Graph of P against θ shows that gas pressure increases INTEGRATION OF
linearly when temperature of the gas rises. At 0°C, gas molecules HISTORY
are still moving and the gas has pressure. At –273°C (absolute
zero), gas molecules no longer move and do not collide with
the walls of the container. Hence, gas pressure becomes zero.
Figure 4.33 shows the graph of P against T.
P / kPa
Joseph Louis Gay-Lussac
(1778–1850) is a French
physicist and chemist who
made quantitative investigation
about the characteristics of
0 T / K gas. He also investigated the
magnetic fi eld of the Earth and
Figure 4.33 Graph of P against T composition of the atmosphere
at high altitudes. In addition, he
Graph of P against T of gas is a straight line through the found two new elements, boron
origin. Th is shows that gas pressure is directly proportional to and iodine.
absolute temperature.
Gay-Lussac’s Law states that pressure is directly proportional
to absolute temperature of a fi xed mass of gas at constant http://bt.sasbadi.com/p4160a
volume.
P ∝ T
P = kT Gay-Lussac’s Law
where k is a constant
P = pressure (Pa)
T = absolute temperature (K) http://bt.sasbadi.com/p4160b
P
As such, = k
T
SMART
If a gas experiences change in pressure and temperature SMART INFO
from condition 1 to condition 2,
P P 1 For Gay-Lussac’s Law, volume
since = k, condition 1 of gas: = k is constant.
T T 1
P 2 P
condition 2 of gas: = k
T
P 1 P 2 2 T
therefore, =
T 1 T 2
P
T = constant
P 1 P 2
T 1 = T 2
160
160 4.4.4
4.4.4