Page 309 - (DK) The Ultimate Visual Dictionary 2nd Ed.
P. 309
THE VARIETY OF MATTER
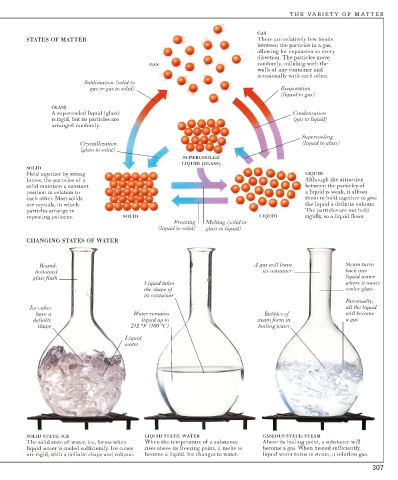
GAS
STATES OF MATTER There are relatively few bonds
between the particles in a gas,
allowing for expansion in every
direction. The particles move
GAS randomly, colliding with the
walls of any container and
occasionally with each other.
Sublimation (solid to
gas or gas to solid) Evaporation
(liquid to gas)
GLASS
A supercooled liquid (glass) Condensation
is rigid, but its particles are (gas to liquid)
arranged randomly.
Supercooling
Crystallization (liquid to glass)
(glass to solid)
SUPERCOOLED
LIQUID (GLASS)
SOLID
Held together by strong LIQUID
forces, the particles of a Although the attraction
solid maintain a constant between the particles of
position in relation to a liquid is weak, it allows
each other. Most solids them to hold together to give
are crystals, in which the liquid a definite volume.
particles arrange in The particles are not held
repeating patterns. SOLID LIQUID rigidly, so a liquid flows.
Freezing Melting (solid or
(liquid to solid) glass to liquid)
CHANGING STATES OF WATER
Round- A gas will leave Steam turns
bottomed its container back into
glass flask liquid water
Liquid takes where it meets
the shape of cooler glass
its container
Eventually,
Ice cubes all the liquid
have a Water remains Bubbles of will become
definite liquid up to steam form in a gas
shape 212 °F (100 °C) boiling water
Liquid
water
SOLID STATE: ICE LIQUID STATE: WATER GASEOUS STATE: STEAM
The solid state of water, ice, forms when When the temperature of a substance Above its boiling point, a substance will
liquid water is cooled sufficiently. Ice cubes rises above its freezing point, it melts to become a gas. When heated sufficiently,
are rigid, with a definite shape and volume. become a liquid. Ice changes to water. liquid water turns to steam, a colorless gas.
307