Page 312 - (DK) The Ultimate Visual Dictionary 2nd Ed.
P. 312
PHYSICS AND CHEMISTR Y
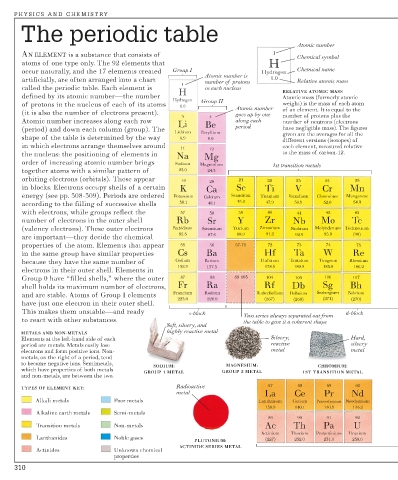
The periodic table
Atomic number
AN ELEMENT is a substance that consists of 1 Chemical symbol
atoms of one type only. The 92 elements that H
occur naturally, and the 17 elements created Group I Hydrogen Chemical name
Atomic number is
artificially, are often arranged into a chart number of protons 1.0 Relative atomic mass
1
called the periodic table. Each element is H in each nucleus RELATIVE ATOMIC MASS
defined by its atomic number—the number Atomic mass (formerly atomic
Hydrogen Group II
of protons in the nucleus of each of its atoms 1.0 Atomic number weight) is the mass of each atom
(it is also the number of electrons present). goes up by one of an element. It is equal to the
3 4 number of protons plus the
Atomic number increases along each row Li Be along each number of neutrons (electrons
(period) and down each column (group). The period have negligible mass). The figures
Lithium Beryllium given are the averages for all the
shape of the table is determined by the way 6.9 9.0
different versions (isotopes) of
in which electrons arrange themselves around 11 12 each element, measured relative
the nucleus: the positioning of elements in Na Mg to the mass of carbon-12.
order of increasing atomic number brings Sodium Magnesium 1st transition metals
together atoms with a similar pattern of 23.0 24.3
orbiting electrons (orbitals). These appear 19 20 21 22 23 24 25
in blocks. Electrons occupy shells of a certain K Ca Sc Ti V Cr Mn
energy (see pp. 308-309). Periods are ordered Potassium Calcium Scandium Titanium Vanadium Chromium Manganese
according to the filling of successive shells 39.1 40.1 45.0 47.9 50.9 52.0 54.9
with electrons, while groups reflect the 37 38 39 40 41 42 43
number of electrons in the outer shell Rb Sr Y Zr Nb Mo Tc
(valency electrons). These outer electrons Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium
are important—they decide the chemical 85.5 87.6 88.9 91.2 92.9 95.9 (98)
properties of the atom. Elements that appear 55 56 57-71 72 73 74 75
in the same group have similar properties Cs Ba Hf Ta W Re
because they have the same number of Cesium Barium Hafnium Tantalum Tungsten Rhenium
132.9 137.3 178.5 180.9 183.8 186.2
electrons in their outer shell. Elements in
Group 0 have “filled shells,” where the outer 87 88 89-103 104 105 106 107
shell holds its maximum number of electrons, Fr Ra Rf Db Sg Bh
and are stable. Atoms of Group I elements Francium Radium Rutherfordium Dubnium Seaborgium Bohrium
223.0 226.0 (267) (268) (271) (270)
have just one electron in their outer shell.
This makes them unstable—and ready s-block Two series always separated out from d-block
to react with other substances. the table to give it a coherent shape
Soft, silvery, and
highly reactive metal
METALS AND NON-METALS
Elements at the left-hand side of each Silvery, Hard,
period are metals. Metals easily lose reactive silvery
electrons and form positive ions. Non- metal metal
metals, on the right of a period, tend
to become negative ions. Semimetals, SODIUM: MAGNESIUM: CHROMIUM:
which have properties of both metals GROUP 1 METAL GROUP 2 METAL 1ST TRANSITION METAL
and non-metals, are between the two.
TYPES OF ELEMENT KEY: Radioactive 57 58 59 60
metal La Ce Pr Nd
Alkali metals Poor metals Lanthanum Cerium Praseodymium Neodymium
138.9 140.1 140.9 144.2
Alkaline earth metals Semi-metals
89 90 91 92
Transition metals Non-metals Ac Th Pa U
Actinium Thorium Protactinium Uranium
Lanthanides Noble gases (227) 232.0 231.0 238.0
PLUTONIUM:
ACTINIDE SERIES METAL
Actinides Unknown chemical
properties
310