Page 310 - (DK) The Ultimate Visual Dictionary 2nd Ed.
P. 310
PHYSICS AND CHEMISTR Y
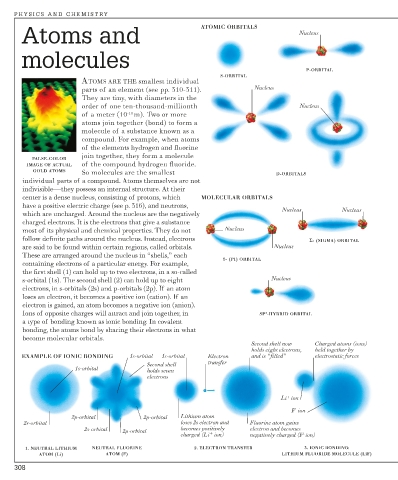
Atoms and ATOMIC ORBITALS Nucleus
molecules
P-ORBITAL
S-ORBITAL
ATOMS ARE THE smallest individual
parts of an element (see pp. 310-311). Nucleus
They are tiny, with diameters in the
order of one ten-thousand-millionth Nucleus
of a meter (10 m). Two or more
-10
atoms join together (bond) to form a
molecule of a substance known as a
compound. For example, when atoms
of the elements hydrogen and fluorine
join together, they form a molecule
FALSE-COLOR
IMAGE OF ACTUAL of the compound hydrogen fluoride.
GOLD ATOMS So molecules are the smallest D-ORBITALS
individual parts of a compound. Atoms themselves are not
indivisible—they possess an internal structure. At their
center is a dense nucleus, consisting of protons, which MOLECULAR ORBITALS
have a positive electric charge (see p. 316), and neutrons,
Nucleus Nucleus
which are uncharged. Around the nucleus are the negatively
charged electrons. It is the electrons that give a substance
most of its physical and chemical properties. They do not Nucleus
follow definite paths around the nucleus. Instead, electrons Σ- (SIGMA) ORBITAL
are said to be found within certain regions, called orbitals. Nucleus
These are arranged around the nucleus in “shells,” each
π- (PI) ORBITAL
containing electrons of a particular energy. For example,
the first shell (1) can hold up to two electrons, in a so-called
s-orbital (1s). The second shell (2) can hold up to eight Nucleus
electrons, in s-orbitals (2s) and p-orbitals (2p). If an atom
loses an electron, it becomes a positive ion (cation). If an
electron is gained, an atom becomes a negative ion (anion).
Ions of opposite charges will attract and join together, in SP -HYBRID ORBITAL
3
a type of bonding known as ionic bonding. In covalent
bonding, the atoms bond by sharing their electrons in what
become molecular orbitals.
Second shell now Charged atoms (ions)
holds eight electrons, held together by
EXAMPLE OF IONIC BONDING 1s-orbital 1s-orbital Electron and is “filled” electrostatic forces
transfer
Second shell
1s-orbital holds seven
electrons
Li + ion
F - ion
2p-orbital 2p-orbital Lithium atom
2s-orbital loses 2s electron and Fluorine atom gains
2s-orbital 2p-orbital becomes positively electron and becomes
+
charged (Li ion) negatively charged (F - ion)
1. NEUTRAL LITHIUM NEUTRAL FLUORINE 2. ELECTRON TRANSFER 3. IONIC BONDING:
ATOM (Li) ATOM (F) LITHIUM FLUORIDE MOLECULE (LiF)
308