Page 119 - Nilam_Publication_module_Chemistry_Form.pdf
P. 119
Chemistry Form 4 • MODULE
CHEMICAL PROPERTIES OF ACID / SIFAT-SIFAT KIMIA ASID
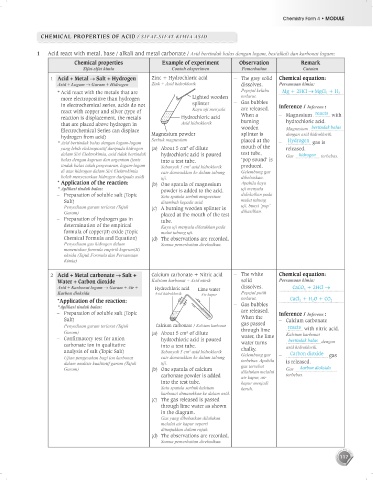
1 Acid react with metal, base / alkali and metal carbonate / Asid bertindak balas dengan logam, bes/alkali dan karbonat logam:
Chemical properties Example of experiment Observation Remark
Sifat-sifat kimia Contoh eksperimen Pemerhatian Catatan
1 Acid + Metal Salt + Hydrogen Zinc + Hydrochloric acid – The grey solid Chemical equation:
Asid + Logam Garam + Hidrogen Zink + Asid hidroklorik dissolves. Persamaan kimia:
* Acid react with the metals that are Pepejal kelabu Mg + 2HCl MgCl 2 + H 2
more electropositive than hydrogen Lighted wooden terlarut.
in electrochemical series, acids do not splinter – Gas bubbles Inference / Inferens :
react with copper and silver (type of Kayu uji menyala are released. reacts
reaction is displacement, the metals Hydrochloric acid When a – Magnesium with
that are placed above hydrogen in Asid hidroklorik burning hydrochloric acid.
Electrochemical Series can displace wooden Magnesium bertindak balas
hydrogen from acid) Magnesium powder splinter is dengan asid hidroklorik.
* Asid bertindak balas dengan logam-logam Serbuk magnesium placed at the – Hydrogen gas is
3
yang lebih elektropositif daripada hidrogen (a) About 5 cm of dilute mouth of the released.
dalam Siri Elektrokimia, asid tidak bertindak hydrochloric acid is poured test tube, Gas hidrogen terbebas.
balas dengan kuprum dan argentum (jenis into a test tube. ‘pop sound’ is
tindak balas ialah penyesaran, logam-logam Sebanyak 5 cm asid hidroklorik produced.
3
di atas hidrogen dalam Siri Elektrokimia cair dimasukkan ke dalam tabung Gelembung gas
boleh menyesarkan hidrogen daripada asid) uji. dibebaskan.
* Application of the reaction: (b) One spatula of magnesium Apabila kayu
* Aplikasi tindak balas: powder is added to the acid. uji menyala
– Preparation of soluble salt (Topic Satu spatula serbuk magnesium didekatkan pada
Salt) ditambah kepada asid. mulut tabung
Penyediaan garam terlarut (Tajuk (c) A burning wooden splinter is uji, bunyi ‘pop’
Garam) placed at the mouth of the test dihasilkan.
– Preparation of hydrogen gas in tube.
determination of the empirical Kayu uji menyala diletakkan pada
formula of copper(II) oxide (Topic mulut tabung uji.
Chemical Formula and Equation) (d) The observations are recorded.
Penyediaan gas hidrogen dalam Semua pemerhatian direkodkan.
menentukan formula empirik kuprum(II)
oksida (Tajuk Formula dan Persamaan
Kimia)
2 Acid + Metal carbonate Salt + Calcium carbonate + Nitric acid – The white Chemical equation:
Water + Carbon dioxide Kalsium karbonat + Asid nitrik solid Persamaan kimia:
Asid + Karbonat logam Garam + Air + Hydrochloric acid Lime water dissolves. CaCO 3 + 2HCl
Karbon dioksida Asid hidroklorik Air kapur Pepejal putih
*Application of the reaction: terlarut. CaCl 2 + H 2 O + CO 2
*Aplikasi tindak balas: – Gas bubbles
– Preparation of soluble salt (Topic are released. Inference / Inferens :
Salt) When the – Calcium carbonate
Penyediaan garam terlarut (Tajuk Calcium carbonate / Kalsium karbonat gas passed reacts with nitric acid.
through lime
3
Garam) (a) About 5 cm of dilute Kalsium karbonat
– Confirmatory test for anion hydrochloric acid is poured water, the lime bertindak balas dengan
carbonate ion in qualitative into a test tube. water turns asid hidroklorik.
analysis of salt (Topic Salt) Sebanyak 5 cm asid hidroklorik chalky. Carbon dioxide
3
Ujian pengesahan bagi ion karbonat cair dimasukkan ke dalam tabung Gelembung gas – gas
terbebas. Apabila
dalam analisis kualitatif garam (Tajuk uji. gas tersebut is released.
Garam) (b) One spatula of calcium dilalukan melalui Gas karbon dioksida
carbonate powder is added air kapur, air terbebas.
into the test tube. kapur menjadi
Satu spatula serbuk kalsium keruh.
karbonat dimasukkan ke dalam asid.
(c) The gas released is passed
through lime water as shown
in the diagram.
Gas yang dibebaskan dilalukan
melalui air kapur seperti
ditunjukkan dalam rajah.
(d) The observations are recorded.
Semua pemerhatian direkodkan.
117
Nilam Publication Sdn. Bhd.
06-Chem F4 (3P).indd 117 12/9/2011 5:55:53 PM