Page 120 - Nilam_Publication_module_Chemistry_Form.pdf
P. 120
MODULE • Chemistry Form 4
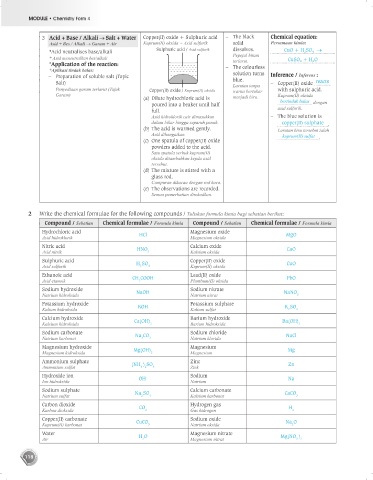
3 Acid + Base / Alkali Salt + Water Copper(II) oxide + Sulphuric acid – The black Chemical equation:
Asid + Bes / Alkali Garam + Air Kuprum(II) oksida + Asid sulfurik solid Persamaan kimia:
*Acid neutralises base/alkali Sulphuric acid / Asid sulfurik dissolves. CuO + H 2 SO 4
* Asid meneutralkan bes/alkali Pepejal hitam CuSO 4 + H 2 O
*Application of the reaction: – terlarut.
The colourless
*Aplikasi tindak balas: solution turns
– Preparation of soluble salt (Topic blue. Inference / Inferens :
Salt) – Copper(II) oxide reacts
Penyediaan garam terlarut (Tajuk Copper(II) oxide / Kuprum(II) oksida Larutan tanpa with sulphuric acid.
warna bertukar
Garam) Kuprum(II) oksida
(a) Dilute hydrochloric acid is menjadi biru.
poured into a beaker until half bertindak balas dengan
full. asid sulfurik.
Asid hidroklorik cair dimasukkan – The blue solution is
dalam bikar hingga separuh penuh. copper(II) sulphate .
(b) The acid is warmed gently. Larutan biru tersebut ialah
Asid dihangatkan. kuprum(II) sulfat
(c) One spatula of copper(II) oxide .
powders added to the acid.
Satu spatula serbuk kuprum(II)
oksida ditambahkan kepda asid
tersebut.
(d) The mixture is stirred with a
glass rod.
Campuran dikacau dengan rod kaca.
(e) The observations are recorded.
Semua pemerhatian direkodkan.
2 Write the chemical formulae for the following compounds / Tuliskan formula kimia bagi sebatian berikut:
Compound / Sebatian Chemical formulae / Formula kimia Compound / Sebatian Chemical formulae / Formula kimia
Hydrochloric acid HCl Magnesium oxide MgO
Asid hidroklorik Magnesium oksida
Nitric acid HNO Calcium oxide CaO
Asid nitrik 3 Kalsium oksida
Sulphuric acid H SO Copper(II) oxide CuO
Asid sulfurik 2 4 Kuprum(II) oksida
Ethanoic acid CH COOH Lead(II) oxide PbO
Asid etanoik 3 Plumbum(II) oksida
Sodium hydroxide NaOH Sodium nitrate NaNO
Natrium hidroksida Natrium nitrat 3
Potassium hydroxide KOH Potassium sulphate K SO
Kalium hidroksida Kalium sulfat 2 4
Calcium hydroxide Ca(OH) Barium hydroxide Ba(OH)
Kalsium hidroksida 2 Barium hidroksida 2
Sodium carbonate Na CO Sodium chloride NaCl
Natrium karbonat 2 3 Natrium klorida
Magnesium hydroxide Mg(OH) Magnesium Mg
Magnesium hidroksida 2 Magnesium
Ammonium sulphate (NH ) SO Zinc Zn
Ammonium sulfat 4 2 4 Zink
Hydroxide ion OH – Sodium Na
Ion hidroksida Natrium
Sodium sulphate Na SO Calcium carbonate CaCO
Natrium sulfat 2 4 Kalsium karbonat 3
Carbon dioxide CO Hydrogen gas H
Karbon dioksida 2 Gas hidrogen 2
Copper(II) carbonate CuCO Sodium oxide Na O
Kuprum(II) karbonat 3 Natrium oksida 2
Water H O Magnesium nitrate Mg(NO )
Air 2 Magnesium nitrat 3 2
118
Nilam Publication Sdn. Bhd.
06-Chem F4 (3P).indd 118 12/9/2011 5:55:54 PM