Page 161 - Nilam_Publication_module_Chemistry_Form.pdf
P. 161
Chemistry Form 4 • MODULE
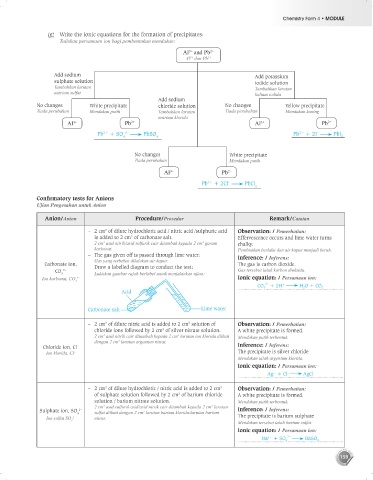
(g) Write the ionic equations for the formation of precipitates:
Tuliskan persamaan ion bagi pembentukan mendakan:
Al and Pb 2+
3+
Al dan Pb 2+
3+
Add sodium Add potassium
sulphate solution iodide solution
Tambahkan larutan Tambahkan larutan
natrium sulfat kalium iodida
Add sodium
No changes White precipitate chloride solution No changes Yellow precipitate
Tiada perubahan Mendakan putih Tambahkan larutan Tiada perubahan Mendakan kuning
natrium klorida
Al 3+ Pb 2+ Al 3+ Pb 2+
Pb + SO PbSO 4 Pb + 2I PbI 2
2+
–
2+
2–
4
No changes White precipitate
Tiada perubahan Mendakan putih
Al 3+ Pb 2+
Pb + 2Cl PbCl
2+
–
2
Conf rmatory tests for Anions
Ujian Pengesahan untuk Anion
Anion/Anion Procedure/Prosedur Remark/Catatan
– 2 cm of dilute hydrochloric acid / nitric acid /sulphuric acid Observation: / Pemerhatian:
3
is added to 2 cm of carbonate salt. Effervescence occurs and lime water turns
3
2 cm asid nitrik/asid sulfurik cair ditambah kepada 2 cm garam chalky.
3
3
karbonat. Pembuakan berlaku dan air kapur menjadi keruh.
– The gas given off is passed through lime water: Inference: / Inferens:
Gas yang terbebas dilalukan air kapur.
Carbonate ion, Draw a labelled diagram to conduct the test: The gas is carbon dioxide.
CO 2– Gas tersebut ialah karbon dioksida.
3 Lukiskan gambar rajah berlabel untuk menjalankan ujian:
Ion karbonat, CO 3 2– Ionic equation: / Persamaan ion:
2–
CO 3 + 2H H 2 O + CO 2
+
Acid
Carbonate salt Lime water
– 2 cm of dilute nitric acid is added to 2 cm solution of Observation: / Pemerhatian:
3
3
chloride ions followed by 2 cm of silver nitrate solution. A white precipitate is formed.
3
2 cm asid nitrik cair ditambah kepada 2 cm larutan ion klorida diikuti Mendakan putih terbentuk.
3
3
3
dengan 2 cm larutan argentum nitrat.
Chloride ion, Cl – Inference: / Inferens:
Ion klorida, Cl – The precipitate is silver chloride
Mendakan ialah argentum klorida.
Ionic equation: / Persamaan ion:
Ag + Cl AgCl
–
+
3
– 2 cm of dilute hydrochloric / nitric acid is added to 2 cm Observation: / Pemerhatian:
3
of sulphate solution followed by 2 cm of barium chloride A white precipitate is formed.
3
solution / barium nitrate solution. Mendakan putih terbentuk.
3
3
2 cm asid sulfurik asid/asid nitrik cair ditambah kepada 2 cm larutan
Sulphate ion, SO 2– 3 Inference: / Inferens:
4 sulfat diikuti dengan 2 cm larutan barium klorida/larutan barium The precipitate is barium sulphate
Ion sulfat SO 2– nitrat.
4
Mendakan tersebut ialah barium sulfat.
Ionic equation: / Persamaan ion:
2–
Ba + SO 4 BaSO 4
2+
159
Nilam Publication Sdn. Bhd.
07-Chem F4 (3p).indd 159 12/9/2011 5:55:24 PM