Page 156 - Nilam_Publication_module_Chemistry_Form.pdf
P. 156
MODULE • Chemistry Form 4
Action of Heat on Salt / Kesan Haba ke atas Garam
1 Some salts decompose when they are heated:
Beberapa jenis garam terurai apabila dipanaskan:
Salt metal oxide + gas
Garam oksida logam gas
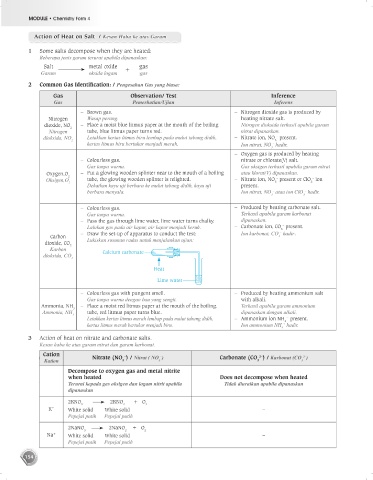
2 Common Gas Identif cation: / Pengesahan Gas yang biasa:
Gas Observation/ Test Inference
Gas Pemerhatian/Ujian Inferens
– Brown gas. – Nitrogen dioxide gas is produced by
Nitrogen Wasap perang. heating nitrate salt.
dioxide, NO 2 – Place a moist blue litmus paper at the mouth of the boiling Nitrogen dioksida terhasil apabila garam
Nitrogen tube, blue litmus paper turns red. nitrat dipanaskan.
–
dioksida, NO Letakkan kertas litmus biru lembap pada mulut tabung didih, – Nitrate ion, NO present.
2 3
kertas litmus biru bertukar menjadi merah. Ion nitrat, NO hadir.
–
3
– Oxygen gas is produced by heating
– Colourless gas. nitrate or chlorate(V) salt.
Gas tanpa warna. Gas oksigen terhasil apabila garam nitrat
Oxygen,O 2 – Put a glowing wooden splinter near to the mouth of a boiling atau klorat(V) dipanaskan.
–
–
Oksigen,O tube, the glowing wooden splinter is relighted. – Nitrate ion, NO present or ClO ion
3
3
2
Dekatkan kayu uji berbara ke mulut tabung didih, kayu uji present.
berbara menyala. Ion nitrat, NO atau ion ClO hadir.
–
–
3 3
– Colourless gas. – Produced by heating carbonate salt.
Gas tanpa warna. Terhasil apabila garam karbonat
– Pass the gas through lime water, lime water turns chalky. dipanaskan.
–
Lalukan gas pada air kapur, air kapur menjadi keruh. – Carbonate ion, CO present.
3
– Draw the set-up of apparatus to conduct the test: Ion karbonat, CO hadir.
–
Carbon 3
dioxide, CO 2 Lukiskan susunan radas untuk menjalankan ujian:
Karbon Calcium carbonate
dioksida, CO
2
Heat
Lime water
– Colourless gas with pungent smell. – Produced by heating ammonium salt
Gas tanpa warna dengan bau yang sengit. with alkali.
Ammonia, NH 3 – Place a moist red litmus paper at the mouth of the boiling. Terhasil apabila garam ammonium
Ammonia, NH tube, red litmus paper turns blue. dipanaskan dengan alkali.
3
Letakkan kertas litmus merah lembap pada mulut tabung didih, – Ammonium ion NH present.
+
4
kertas litmus merah bertukar menjadi biru. Ion ammonium NH hadir.
+
4
3 Action of heat on nitrate and carbonate salts.
Kesan haba ke atas garam nitrat dan garam karbonat.
Cation – – 2– 2–
Kation Nitrate (NO ) / Nitrat ( NO ) Carbonate (CO ) / Karbonat (CO )
3
3
3
3
Decompose to oxygen gas and metal nitrite
when heated Does not decompose when heated
Terurai kepada gas oksigen dan logam nitrit apabila Tidak diuraikan apabila dipanaskan
dipanaskan
2KNO 2KNO + O
3 2 2
K + White solid White solid –
Pepejal putih Pepejal putih
2NaNO 2NaNO + O
3 2 2
Na + White solid White solid –
Pepejal putih Pepejal putih
154
Nilam Publication Sdn. Bhd.
07-Chem F4 (3p).indd 154 12/9/2011 5:55:23 PM