Page 5 - Nilam_Publication_module_Chemistry_Form.pdf
P. 5
Chemistry Form 4 • MODULE
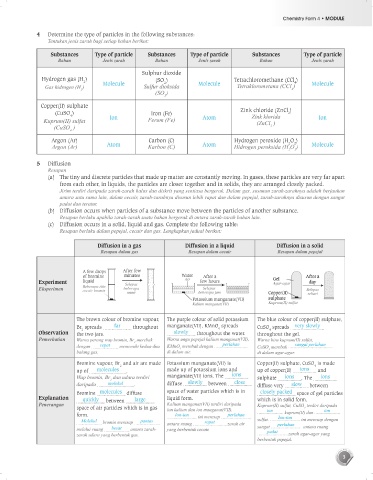
4 Determine the type of particles in the following substances:
Tentukan jenis zarah bagi setiap bahan berikut:
Substances Type of particle Substances Type of particle Substances Type of particle
Bahan Jenis zarah Bahan Jenis zarah Bahan Jenis zarah
Sulphur dioxide
Hydrogen gas (H ) (SO ) Tetrachloromethane (CCl )
2 Molecule 2 Molecule 4 Molecule
Gas hidrogen (H ) Sulfur dioksida Tetraklorometana (CCl )
2 4
(SO )
2
Copper(II) sulphate
(CuSO ) Iron (Fe) Zink chloride (ZnCl )
2
4 Ion Atom Zink klorida Ion
Kuprum(II) sulfat Ferum (Fe) (ZnCl )
(CuSO ) 2
4
Argon (Ar) Carbon (C) Hydrogen peroxide (H O )
2
2
Argon (Ar) Atom Karbon (C) Atom Hidrogen peroksida (H O ) Molecule
2 2
5 Diffusion
Resapan
(a) The tiny and discrete particles that made up matter are constantly moving. In gases, these particles are very far apart
from each other, in liquids, the particles are closer together and in solids, they are arranged closely packed.
Jirim terdiri daripada zarah-zarah halus dan diskrit yang sentiasa bergerak. Dalam gas, susunan zarah-zarahnya adalah berjauhan
antara satu sama lain, dalam cecair, zarah-zarahnya disusun lebih rapat dan dalam pepejal, zarah-zarahnya disusun dengan sangat
padat dan teratur.
(b) Diffusion occurs when particles of a substance move between the particles of another substance.
Resapan berlaku apabila zarah-zarah suatu bahan bergerak di antara zarah-zarah bahan lain.
(c) Diffusion occurs in a solid, liquid and gas. Complete the following table:
Resapan berlaku dalam pepejal, cecair dan gas. Lengkapkan jadual berikut:
Diffusion in a gas Diffusion in a liquid Diffusion in a solid
Resapan dalam gas Resapan dalam cecair Resapan dalam pepejal
A few drops After few
of bromine minutes Water After a After a
Gel
Experiment liquid Selepas Air few hours Agar-agar day
Eksperimen Beberapa titis beberapa Selepas Selepas
cecair bromin
minit beberapa jam Copper(II) sehari
Potassium manganate(VII) sulphate
Kalium manganat(VII) Kuprum(II) sulfat
The brown colour of bromine vapour, The purple colour of solid potassium The blue colour of copper(II) sulphate,
Br spreads far throughout manganate(VII), KMnO spreads CuSO spreads very slowly
4
4
2
Observation the two jars. slowly throughout the water. throughout the gel.
Pemerhatian Warna perang wap bromin, Br merebak Warna ungu pepejal kalium manganat(VII), Warna biru kuprum(II) sulfat,
2
dengan cepat memenuhi kedua-dua KMnO merebak dengan perlahan CuSO merebak sangat perlahan
4
4
balang gas. di dalam air. di dalam agar-agar.
Bromine vapour, Br and air are made Potassium manganate(VII) is Copper(II) sulphate, CuSO is made
4
2
up of molecules . made up of potassium ions and up of copper(II) ions and
Wap bromin, Br dan udara terdiri manganate(VII) ions. The ions sulphate ions . The ions
2
daripada molekul . diffuse slowly between close diffuse very slow between
Bromine molecules diffuse space of water particles which is in closely packed space of gel particles
Explanation quickly between large liquid form. which is in solid form.
Penerangan Kalium manganat(VII) terdiri daripada Kuprum(II) sulfat, CuSO terdiri daripada
space of air particles which is in gas ion kalium dan ion manganat(VII). ion 4 ion
kuprum(II) dan
form. Ion-ion ini meresap perlahan Ion-ion
Molekul bromin meresap pantas antara ruang rapat zarah air sulfat. perlahan ini meresap dengan
melalui ruang besar antara zarah- yang berbentuk cecair. sangat antara ruang
padat
zarah udara yang berbentuk gas. zarah agar-agar yang
berbentuk pepejal.
3
Nilam Publication Sdn. Bhd.
01-Chem F4 (3p).indd 3 12/9/2011 5:59:28 PM