Page 671 - APPLIED PROCESS DESIGN FOR CHEMICAL AND PETROCHEMICAL PLANTS, Volume 1, 3rd Edition
P. 671
Appendix 619
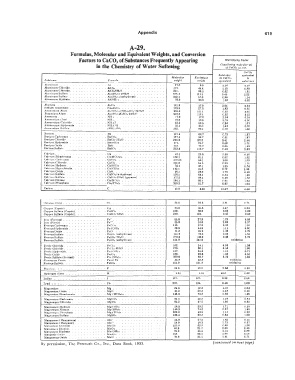
A-29.
Formulas, Molecular and Equivalent Weights, and Conversion
Factors to CaC0 3 of Substances Frequently Appearing /l!ultipl11ing Factor
in the Chemistry of Water Softening ('onAir/criMg ,norte'lrar 1L"f,
of CaC01 a11 I fJO.
csco,
Sub11tnnc� �qviPUl#nl
lt,loluular Eq11ito(r.nl to CaCO, to
S11halunl"e For111ulrt u•tight u·rir;ht �quiral,n: euh,t,111 r �
--
Aluminum Al 27.0 9.0 6.illl O.IR
Aluminum C.hlnrid"'. AIClt 131. H.4 1.13 O.R9
Aluminum Chloride. AIC1,.RHtO. 241. R0.5 0.62 J.61
Aluminum Sulfer e Al.fSO.)l· JRlltO 6f;l'i . .t II I.I 0.45 2.22
Aluminum SuH11t.n Al.fSO,ll (•nhydrou11) . 342.1 67.0 O.RR 1.14
Aluminum Jlydr&l8. Al(OII), . 78.0 26.0 l.9t 0.62
-··-
Alumin11 Al,O, 101.9 17.0 2.94 O.S4
Sodium Aturninatc . N11.1Al11,, 163.9 27.1 l.83 0.65
Amrnnniurn Alurr. AltfSO,l 1 (NH,>,S0,·24Ht0 906.6 151.1 0.33 S-02
.Pota�i urn A I um . Al1(SO,l1K,SO,· 20110 94R.R 156. t 0.32 3.12
Arnmc)nia . NH, 17.0 17.0 2.94 0.34
Ammoniurr. (l,.m) Niie 18.0 IR.O 2.78 0.36
Amn1nnium C:hl(>rid,• . Nll1CI. 63.5 63.5 0.94 J.07
Ammonium Hyd roxide NJI,c>ll 35.l 35.1 1.43 0.70
Amrncmlum Su1C11tc (Nll1)rSO, 132. 66,1 0.76 1.s2
Har-iurn II• 137.4 6R.7 0.73 !.S7
Barium Carhnnate flllC01. 197.4 9R. 7 0.61 !.97
Buit.m C.hlorldr . fleC:lt·21J,O 24-1.3 122.2 {l.4i 2.H
Baricrn Hydroxide 1111.(0ll}t l 71. R5.7 0.69 1- 71
flariuni Chdrte nso. J !'.13. 76.7 0.65 U3
Barium Sulrat,! BaS(), 233.4 IJG. 7 C.43 2.33
r.11.1 ... ium. (;a 40.! 20.0 2.60 0.40
Calt-hJrn nit'arhnnale C11.(HC::O,h. 162. l 81.1 0.62 J.62
C.:ald:.tm C:RrhonP.te CaC01. JOO.OB 60.1 I.DO 1.00
<:al<"iurn Chlorfde . CoCli lll.O 65.5 0.90 1.11
t"alcium llydrMc. Ca(OIJ1, 74.1 37.1 1.35 0.74
Cal<"ium llypochlorltft. C•(CIUJt. lUI 35.R 0.70 1.•a
Cakium Oxide . Cao. b<i.J 2R.O J. 79 0.66
Cal<"ium �ulfarn CaSO, (anhydroU11) 136.1 68.1 0.74 1-36
Calriurn Sulfate CL.�0,·211,0 (iYPBUm) 172.2 86.1 0.68 1.12
C:alr!um Nitrate Ca(NO,), 164.J 82.1 0.61 1.64
f-- � :aleiurn Phosphate Ca,rJ'01)1 310.3 61.7 0.97 1.D3
' Carbnn C. 12.0 S.00 16.67 0.06
Cl,lnrin•• (Ion) Cl. 36.6 S6.6 0.11
C•111pl"r (Copri<"J Cu 6�.6 Sl.8 1.67 0.64
C.:<lpµrr Su)(ate (Cupric). CuS01 !GO. RO.O 0.63 J.60
Ct>ppN SuJ(ale (Cupric). CuSOc·SII.() 260. 125. 0.40 2-60
Irnn ( Frrrou11) J-'P.''. 65.8 27.9 1. 79 0.66
lrc•n ( F1Hri1') F�"'. 55.8 18.6 2.69 0.37
Ferrous Catbonete i'"P.f;01. 116. 57.9 0.86 1.16
Ferrnua HydroJ1.ide . Fe (OH>, R9.9 44.9 l.ll 0.90
F,•rrou!J (hidn FeO 71.A 35.9 J.39 0.72
Forrnua Sulfate F"SO, (anhydrous) 151.9 76.0 0.66 1-52
Ferrous SuHate Fe�O,· 7H,O 27H.O 139.0 0.36 2. 7K
Ferrous Sulfate. 1-'eSO, (a11hydrm1.'I) 151.9 151.9 oxidation
F,,rric Chloride i''PCl1 162. 5'-1 0.93 J.08
Forric Chloeide . F,�Ct,· 611,0 270. 90.1 0.56 1.80
Frrric Hyd roaido . Fe tOII), 107. 35.6 Ul o. 71
Ferr!c Oxldn },'p1(), 160. 26.6 I.BB 0.63
Fnric SuHal!! (}··Nri11ul) i' 1!t (S0,) 1 399.9 66. 7 0.76 1-33
0
Fert(JU9 or Fr-rrlc Fe or Fe. 65.8 65.8 o:tidation
Ferroue Sulf•Le. r-so.. 151.9 151.9 o:tidation
Fluorine. : : F. 19.0 19.0 2.� 0.3R
Hydr-ogen (Ion) H 1.01 1.01 60.0 0.02
Iudine 127. 127. 0.40 2.64
fA>ad : Pb 207. 104. 0.48 2,08
Magn"111urr. Mi 24.3 12.2 4.10 0.24
40.3
MagnCRium Oxid•! MgO 146.3 20.2 2.4B 0.40
MaKn"11ium Bieerbonete .
-------- Mi (H<:0,1t 84.3 73.2 0.68 J.46
42.2
1.19
MgCO,
O.R4
:\h,:riM1ium Cuh()nate
- Mg Ch 95.2 47.6 1.06 0.95
Mal(nPsium Chloride
�bgnPJ11iurr. J lydra!P. Mi(OHh 5R.3 29.2 1.71 0.68
Ma1,iC11es!um Ni teate . �hc<N(J.lJ ICR.3 74.2 0.67 l-48
Maa,:nPsium Phn�11hat.2 M21!PO,)t. 262.9 U.8 1.14 O.AB
:.20
� la._:ncsium .Sulfalr MgSO, 120.4 60.2 0.R3 0.55
I M an�anf"flP ( M anicank) Mo"' 12!',.R 62.9 2.73 0.37
64.9
27.!')
M11''
1.H2
M an,anr,e IM an,annua)
IA.3
!>4.9
O.RO
J.26
MnCh
M11t11{Anf· � 1> l:hloridP.
I Man�u,wi:e llioxidt>. �{nO,. l 5R. 21.7 2.30 0.4:i
H6.9
·�-0
0.89
Mrd()llh
44.,
1.13
Man�.,,.·,e llyd.ate
0.6�
l.90
MntO,.
26.3
Man1<anic (hidP
o. 71
70.9
MnU
1.41
35.5
ManKanous Oxlde
By permission, The Permutit Co., Inc., Data Book, 1953. ( continued on next page)