Page 672 - APPLIED PROCESS DESIGN FOR CHEMICAL AND PETROCHEMICAL PLANTS, Volume 1, 3rd Edition
P. 672
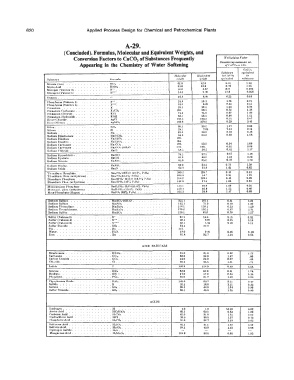
620 Applied Process Design for Chemical and Petrochemical Plants
A-29.
(Concluded). Formulas, Molecular and Equi,alent Weights, and
Conversion Factors to CaC0 3 of Substances Frequently Al11Wpt11i11g Puctor
Appearing in the Chemistry of Water Softening Ccm�idcrl11� moluular wt,
P/ C'uro, nit I 00.
ca.co ..
Sub:d11nre rquirlu.lr11t
,\Juluulu, e',/HirnftHl '" n,,·o. lo
Svb,ranu 1-'uruu,la trf'i11Jtt 1,1·,i11/tal t'!/llil'olf�nl •ll'•�tuHrC
Nilr&lc ( tun) NO, 62.0 62.0 O.Hl 1.24
1.2G
0.79
6:1.0
Nitric Aeid IINO, . 6:1.0 4.67 10.H 0.093
NiLrngen (Vall!nce 3) N'" 14.0 17.9 n.oss
NilrC>¥�n (Vah:nt:P r1) N'"" \4.0 :!.HO
:, 16.0 H.00 s.as 0.16
l'ho�phvru.:t (Vah:ricc :JI I'"'. 31.0 J0.3 ·1.76 0.21
6.20
l'hr�ph1,rua (Vah•m•e fi) . !""". 31.0 39 1 K.33 0.1:!
1.26
0.7H
l 'oUL.!i�i.um . K 3\J.l
I'orasstum Carbonate . K1f:01 l:lli. 69.1 o.n t.3K
Poresaium Chloride . KCI 74.6 7l.6 0.67 1.0
1.12
Potassium Hydroxide . KOU 56, I 66.1 0.8K 2.K7
0.3r;
Sil ver Chloride. A•Ci . 1-1:1.:1 143.3 0.29 a.so
Stlvvr Nit rate AicN01 iss.s J69.9
.Sili<'a .• Sil>. GU.I 30.0 1.67 0.60
1.14
0.14
Silicon • Si :!Ii.I 7.03 2.18 0.46
Sodium. , Na .. 2:1.0 23.0 0.60 J.68
Sodium llkarhunate Nall CO,. H4.0 M.O
Sodium Disulf.&L1:t Na.llSO,. 120.
Sodium Dis.ullitP. NallS01• 104.
1.06
Sodium Carbr.netc Na�C01 lllt:L 63.0 0.94 2.86
SuJium Carlutnillfe N"'1C0,· 10lhO :!t<G. 148. 0.35 1.17
Sodium Chluridu . NaCl !JK . .'.", 5�.5 0.85
Sodium llypl.)(:hloril., . Na\.10. 7-l.;j 37.3 0.67 1.49
0.HO
1.25
Sodium Hydrate NoOll. 40.0 40.0 e.ss
Sodium Nitrate NaN01 KJ.0 k5.0 1.70
Sodium NiLrite. NaNA.>, 69 0 34.5 0.7:l 1.31l
Sodium Oxlde Na,O , 62.0 31.0 1.61 0.62
'fri-sodKun l'hu.:sph&Lt!. Na11'0,·J2H,O (IK.7'1� 1'10,) 3K0.2 126. 7 o to 2.53
1.09
'I'rf-eodlum l'hcs. tauhydruua) Na11'0,(4:l.:!'�i;, · 1'�0,) 164.0 !,,l.7 0.91 2.39
Di-sodium I'hoaphato . . Na1IIP01·121110 (19.R''.� l'·1U,) 3!'1H,t 119.4 0.4�
Di-aodium l'huJt, (:,,nl,ydrouit) Natll i-o, (&0% l'i<>.) . 142.0 47.3 1.06 0.95
Mono-sodium l'ht1!1J1hate NalL!'0,· 1110 (51.4% l 1U,>. 1ax.1 46.0 1.09 0.9'l
1
Mono-eud. phos. (anhydr,,�) NaH�l'O• (:i9.1% 1'10•) l:!O.n 40.0 1.25 IUSO
0.68
Mcta-t•hnllJ)hale (ll•Kan) NaP<h (69% 1',lh) 10�.o 31.0 1.-17
Sodium Sulra1e. . , . , Na,SO,· JOll:0 . 322.1 161.1 0.31 3.22
Sodium Sulfate ... NatSO, H2.1 71.0 0.70 1.42
Sodium Thio�ulraLt!. N•:S20,. l:!H.l 15/i.1 O.G3 1.59
Sodium T1•trathionate. Na1S,Ot. 270.2 135.1 0.87 2.71
Sodium Sulfite Na,801 126.1 K:l.O 0.79 1.27
Sulfur (Valence 2) S" 3". I 16.0 3.f3 0.32
Sulfur ( valence 4) S"" 3:!.l K.O:! 6.25 0.16
SuHur ( Val,•nce 6) S""" az.t 5.34 9.10 0.11
Sullur l>ioxidc . SO,. s,.1 :t:!'.O
Tin .. . Sn • 119.
'Water. 11,0. 1�.o 9 nu 6.56 0.18
Zinc . Zn 65.4 32.7 J.54 0.65
ACID RADI<:Al.S
Dkarbonate . ucu, . 61.0 61.0 0.82 l."2
Carhonatf'-. co.. 60.0 30.0 1.67 .60
Carbon J)iO:Lida CO,. H.O 22.0 2.27 .4-1
Chloride. Cl. 35.5 35,5 1.41 , 71
Iodide 126.9 t:W.9 0.40 2.64
Nltrate NO, 62.0 6t.O 0.81 1.24
Hydratu. OH: I 7.0 17.0 2.94 0.34
l'husphatc. r-o.. 95.0 31.7 1.6H 0.63
l'ho�phoroua Oxide l'10,. 142.0 23.7 2.11 0.47
1
Su l1lde . : : .. s. 32.1 16.0 3.11 0.32
Sul late so •. 96.1 4M.O 1.04 0.96
Hutrur Trio•lde. so,. 80.1 40.0 1.26 0.HO
ACIDS
llydru"l!n . . H. , . 1.0 1.0 50.00 0.02
At'..-lic Ai:ld . HC,U.01 60.1 60.1 0.83 1.20
Carbonic Acid . HrC01. 62.0 31.0 1.61 0.62
llydro<:hloric Acid IICI .. 36.5 36.5 1.37 0.73
l'hoat>huric Acid HJl'O,. 9•.o 32. 7 r.ss 0.65
Suttur oua Acid . !liSO,. M2.l 41.1 1.22 0.82
Sulfuric Acid. H�O,. 9K.l 4�.o J.02 0.9H
Hydrogen Sulfidff, H1S. ..
!th11KanoW1 Acid . ll1Mn01. 10,.9 62.6 0.96 1.05