Page 21 - Spotlight A+ Physics Form 4.5
P. 21
Form
5
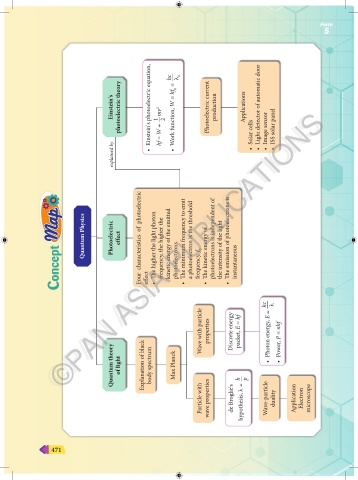
Einstein's photoelectric theory Einstein's photoelectric equation, 1 hf = W + —mv 2 2 hc Work function, W = hf 0 = –— λ 0 Photoelectric current production Applications Light detector of automatic door Image sensor ISS solar panel
©PAN ASIA PUBLICATIONS
©PAN ASIA PUBLICATIONS
explained by • • Solar cells • • • •
Four characteristics of photoelectric The higher the light photon frequency, the higher the kinetic energy of the emitted photoelectrons. The minimum frequency to emit a photoelectron is the threshold frequency, f 0 The kinetic energy of photoelectrons is independent of the intensity of the light The emission of photoelectrons is instantaneous
Quantum Physics
Photoelectric
effect
effect
•
•
•
•
hc λ
Wave with particle properties Discrete energy packet, E = hf Photon energy, E = –— Power, P = nhf
Quantum theory of light Explanation of black body spectrum Max Planck h p • •
Particle with wave properties de Broglie's hypothesis, λ = — Wave-particle duality Application Electron microscope
471