Page 210 - Basic Principles of Textile Coloration
P. 210
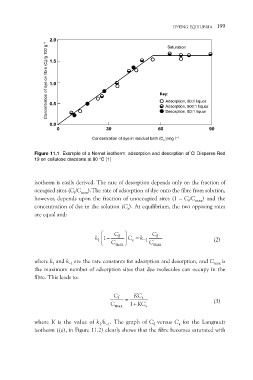
Concentration of dye on fibre (Cf)/g 100 g –1 DYEING EQUILIBRIA 199
2.0
Saturation
1.5
1.0
Key:
0.5 Adsorption, 80:1 liquor
Adsorption, 800:1 liquor
Desorption, 80:1 liquor
0.0 30 60 90
0 Concentration of dye in residual bath (Cs )/mg l–1
Figure 11.1 Example of a Nernst isotherm: adsorption and desorption of CI Disperse Red
19 on cellulose diacetate at 80 °C [1]
isotherm is easily derived. The rate of desorption depends only on the fraction of
occupied sites (Cf/Cmax).The rate of adsorption of dye onto the fibre from solution,
however, depends upon the fraction of unoccupied sites (1 – Cf/Cmax) and the
concentration of dye in the solution (Cs). At equilibrium, the two opposing rates
are equal and:
Ë Cf Û Cs = k-1 Cf (2)
k1 ÌÍ1 - Cmax ÜÝ Cmax
where k1 and k–1 are the rate constants for adsorption and desorption, and Cmax is
the maximum number of adsorption sites that dye molecules can occupy in the
fibre. This leads to:
Cf = KCs (3)
Cmax 1 + KCs
where K is the value of k1/k–1. The graph of Cf versus Cs for the Langmuir
isotherm ((a), in Figure 11.2) clearly shows that the fibre becomes saturated with