Page 211 - Basic Principles of Textile Coloration
P. 211
200 DYEING THEORY
dye when all the available adsorption sites are filled. The maximum number of
sites in the fibre can be determined from the slope of the linear graph of Cs/Cf
versus Cs, according to the equation:
Cs = Cs + 1 (4)
Cf Cmax KCmax
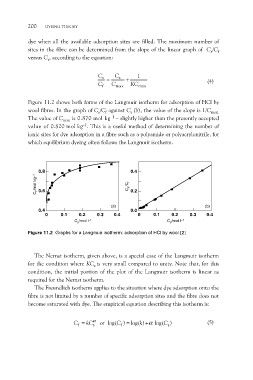
Figure 11.2 shows both forms of the Langmuir isotherm for adsorption of HCl by
wool fibres. In the graph of Cs/Cf against Cs (b), the value of the slope is 1/Cmax.
The value of Cmax is 0.870 mol kg–1 – slightly higher than the presently accepted
value of 0.820 mol kg–1. This is a useful method of determining the number of
ionic sites for dye adsorption in a fibre such as a polyamide or polyacrylonitrile, for
which equilibrium dyeing often follows the Langmuir isotherm.
0.8 0.4
Cf/mol kg–1
Cs /Cf
0.6 0.2
(a) (b)
0.4 0.1 0.2 0.3 0.4 0.0 0.1 0.2 0.3 0.4
0 0
Cs/mol l–1 Cs/mol l–1
Figure 11.2 Graphs for a Langmuir isotherm: adsorption of HCl by wool [2]
The Nernst isotherm, given above, is a special case of the Langmuir isotherm
for the condition where KCs is very small compared to unity. Note that, for this
condition, the initial portion of the plot of the Langmuir isotherm is linear as
required for the Nernst isotherm.
The Freundlich isotherm applies to the situation where dye adsorption onto the
fibre is not limited by a number of specific adsorption sites and the fibre does not
become saturated with dye. The empirical equation describing this isotherm is:
Cf = kCas or log(Cf ) = log(k)+ a log(Cs ) (5)