Page 214 - Basic Principles of Textile Coloration
P. 214
DYEING EQUILIBRIA 203
solution (as). This is difficult and usually molar concentrations must be
substituted. For example, in dyeing a synthetic fibre with a pure non-ionic disperse
dye, at equilibrium:
- Dm 0 = RT ln Ë Cf Û (9)
ÍÌ Cs ÝÜ
The equilibrium constant for dyeing with a dye of given affinity is given by K =
Cf/Cs and depends only on the temperature. This is exactly the situation described
by the Nernst isotherm from which the affinity can be calculated directly with the
assumption that the term Cf/Cs is a correct approximation for the activity quotient
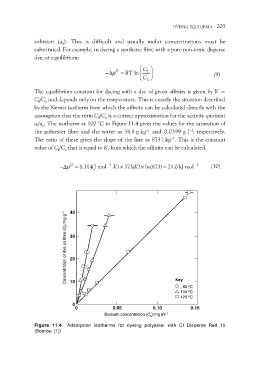
af/as. The isotherm at 100 °C in Figure 11.4 gives the values for the saturation of
the polyester fibre and the water as 34.8 g kg–1 and 0.0399 g l–1, respectively.
The ratio of these gives the slope of the line as 873 l kg–1. This is the constant
value of Cf/Cs that is equal to K, from which the affinity can be calculated.
-Dm0 = 8.314(J mol-1 K ) 373(K) ln(873) = 21.0 kJ mol-1 (10)
Concentration of dye on fibre (Cf)/mg g–1 40
30
20
10 Key
83 oC
100 oC
120 oC
0 0 0.05 0.10 0.15
Dyebath concentration (Cs)/mg ml–1
Figure 11.4 Adsorption isotherms for dyeing polyester with CI Disperse Red 15
(Source: [1])