Page 212 - Basic Principles of Textile Coloration
P. 212
DYEING EQUILIBRIA 201
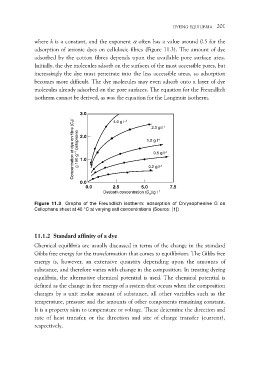
where k is a constant, and the exponent a often has a value around 0.5 for the
adsorption of anionic dyes on cellulosic fibres (Figure 11.3). The amount of dye
adsorbed by the cotton fibres depends upon the available pore surface area.
Initially, the dye molecules adsorb on the surfaces of the most accessible pores, but
increasingly the dye must penetrate into the less accessible areas, so adsorption
becomes more difficult. The dye molecules may even adsorb onto a layer of dye
molecules already adsorbed on the pore surfaces. The equation for the Freundlich
isotherm cannot be derived, as was the equation for the Langmuir isotherm.
3.0
Concentration of dye on fibre (Cf)/ 4.0 g l–1
g 100 g–1 cellophane
2.0 g l–1
2.0
1.0 g l–1
0.5 g l–1
1.0
0.2 g l–1
0.0 2.5 5.0 7.5
0.0 Dyebath concentration (Cs)/g l–1
Figure 11.3 Graphs of the Freundlich isotherm: adsorption of Chrysophenine G on
Cellophane sheet at 40 °C at varying salt concentrations (Source: [1])
11.1.2 Standard affinity of a dye
Chemical equilibria are usually discussed in terms of the change in the standard
Gibbs free energy for the transformation that comes to equilibrium. The Gibbs free
energy is, however, an extensive quantity depending upon the amounts of
substance, and therefore varies with change in the composition. In treating dyeing
equilibria, the alternative chemical potential is used. The chemical potential is
defined as the change in free energy of a system that occurs when the composition
changes by a unit molar amount of substance, all other variables such as the
temperature, pressure and the amounts of other components remaining constant.
It is a property akin to temperature or voltage. These determine the direction and
rate of heat transfer, or the direction and size of charge transfer (current),
respectively.