Page 254 - Basic Principles of Textile Coloration
P. 254
THE APPLICATION OF ACID DYES IN DYEING WOOL 243
weakly acidic baths with a pH above 5. On the other hand, small dye molecules
with many sulphonate groups migrate very well during dyeing and have poor wet
fastness. For low molecular weight acid dyes the dyebath exhaustion is low when
dyeing at a pH greater than 4. Acid dyes therefore show the usual inverse
relationship of migration ability to wet fastness. Table 13.1 summarises the dyeing
properties of the various types of acid dye. Note the difference in molecular
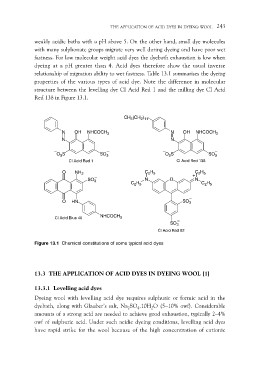
structure between the levelling dye CI Acid Red 1 and the milling dye CI Acid
Red 138 in Figure 13.1.
CH3(CH2)11
N OH NHCOCH3 N OH NHCOCH3
N N
O3S SO3 O3S SO3
CI Acid Red 1 CI Acid Red 138
O NH2 C2H5 C2H5
SO3 N ON
C2H5
C2H5
O HN SO3
CI Acid Blue 40 NHCOCH3
SO3
CI Acid Red 52
Figure 13.1 Chemical constitutions of some typical acid dyes
13.3 THE APPLICATION OF ACID DYES IN DYEING WOOL [1]
13.3.1 Levelling acid dyes
Dyeing wool with levelling acid dye requires sulphuric or formic acid in the
dyebath, along with Glauber’s salt, Na2SO4.10H2O (5–10% owf). Considerable
amounts of a strong acid are needed to achieve good exhaustion, typically 2–4%
owf of sulphuric acid. Under such acidic dyeing conditions, levelling acid dyes
have rapid strike for the wool because of the high concentration of cationic