Page 374 - Basic Principles of Textile Coloration
P. 374
THE REDUCTION OF QUINONE VAT DYES 363
(2) a moderate concentration of NaOH and a lower vatting temperature
(50 °C);
(3) a low concentration of NaOH and a low vatting temperature (30–40 °C).
17.3.4 Redox potentials and rate of vatting
Dyes that are easy to reduce are more difficult to oxidise, and vice versa. The
standard redox potential (E°) gives a measure of the ease of reduction at a given
pH. In strongly alkaline solution, the redox potential E is given by
E = E + RT ln Ë aox Û (1)
2F ÍÌ ared ÝÜ
where R is the gas constant, T the absolute temperature, F the Faraday constant,
and aox and ared are the activities of the oxidised and reduced forms of the vat dye.
The value of E° is related to the standard free energy change for the reduction
process.
ox + 2e- ì red2- (2)
-DG = 2EF = RT ln(K)
500
excess ferricyanide
0
Redox potential/mV –500
–1000 mmol reduced dye
excess hydros
–1500 5 10 15
0
Volume K3Fe(CN)6/ml
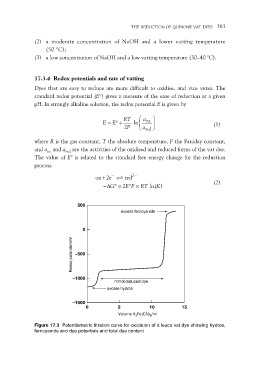
Figure 17.3 Potentiometric titration curve for oxidation of a leuco vat dye showing hydros,
ferricyanide and dye potentials and total dye content