Page 370 - Basic Principles of Textile Coloration
P. 370
CHEMICAL CONSTITUTION OF QUINONE VAT DYES 359
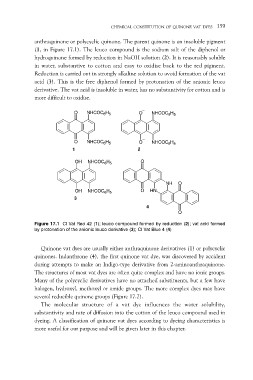
anthraquinone or polycyclic quinone. The parent quinone is an insoluble pigment
(1, in Figure 17.1). The leuco compound is the sodium salt of the diphenol or
hydroquinone formed by reduction in NaOH solution (2). It is reasonably soluble
in water, substantive to cotton and easy to oxidise back to the red pigment.
Reduction is carried out in strongly alkaline solution to avoid formation of the vat
acid (3). This is the free diphenol formed by protonation of the anionic leuco
derivative. The vat acid is insoluble in water, has no substantivity for cotton and is
more difficult to oxidise.
O NHCOC6H5 O NHCOC6H5
O NHCOC6H5 O NHCOC6H5
1 2
OH NHCOC6H5 O
NH O
OH NHCOC6H5 O HN
3
4
O
Figure 17.1 CI Vat Red 42 (1); leuco compound formed by reduction (2); vat acid formed
by protonation of the anionic leuco derivative (3); CI Vat Blue 4 (4)
Quinone vat dyes are usually either anthraquinone derivatives (1) or polycyclic
quinones. Indanthrone (4), the first quinone vat dye, was discovered by accident
during attempts to make an Indigo-type derivative from 2-aminoanthraquinone.
The structures of most vat dyes are often quite complex and have no ionic groups.
Many of the polycyclic derivatives have no attached substituents, but a few have
halogen, hydroxyl, methoxyl or amide groups. The more complex dyes may have
several reducible quinone groups (Figure 17.2).
The molecular structure of a vat dye influences the water solubility,
substantivity and rate of diffusion into the cotton of the leuco compound used in
dyeing. A classification of quinone vat dyes according to dyeing characteristics is
more useful for our purpose and will be given later in this chapter.