Page 412 - Basic Principles of Textile Coloration
P. 412
AZOIC DYES 401
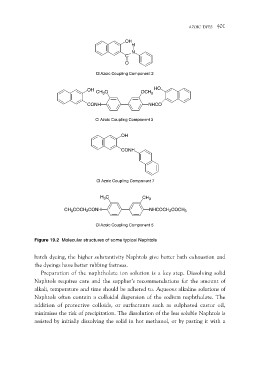
OH
H
N
C
O
Cl Azoic Coupling Component 2
OH CH3O HO
CONH OCH3
NHCO
Cl Azoic Coupling Component 3
OH
CONH
Cl Azoic Coupling Component 7
H3C CH3
CH3COCH2CONH NHCOCH2COCH3
Cl Azoic Coupling Component 5
Figure 19.2 Molecular structures of some typical Naphtols
batch dyeing, the higher substantivity Naphtols give better bath exhaustion and
the dyeings have better rubbing fastness.
Preparation of the naphtholate ion solution is a key step. Dissolving solid
Naphtols requires care and the supplier’s recommendations for the amount of
alkali, temperature and time should be adhered to. Aqueous alkaline solutions of
Naphtols often contain a colloidal dispersion of the sodium naphtholate. The
addition of protective colloids, or surfactants such as sulphated castor oil,
minimises the risk of precipitation. The dissolution of the less soluble Naphtols is
assisted by initially dissolving the solid in hot methanol, or by pasting it with a