Page 64 - Basic Principles of Textile Coloration
P. 64
NYLON FIBRES 53
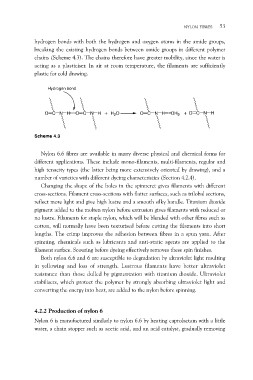
hydrogen bonds with both the hydrogen and oxygen atoms in the amide groups,
breaking the existing hydrogen bonds between amide groups in different polymer
chains (Scheme 4.3). The chains therefore have greater mobility, since the water is
acting as a plasticiser. In air at room temperature, the filaments are sufficiently
plastic for cold drawing.
Hydrogen bond
O C N H O C N H + H2O O C N H OH2 + O C N H
Scheme 4.3
Nylon 6.6 fibres are available in many diverse physical and chemical forms for
different applications. These include mono-filaments, multi-filaments, regular and
high tenacity types (the latter being more extensively oriented by drawing), and a
number of varieties with different dyeing characteristics (Section 4.2.4).
Changing the shape of the holes in the spinneret gives filaments with different
cross-sections. Filament cross-sections with flatter surfaces, such as trilobal sections,
reflect more light and give high lustre and a smooth silky handle. Titanium dioxide
pigment added to the molten nylon before extrusion gives filaments with reduced or
no lustre. Filaments for staple nylon, which will be blended with other fibres such as
cotton, will normally have been texturised before cutting the filaments into short
lengths. The crimp improves the adhesion between fibres in a spun yarn. After
spinning, chemicals such as lubricants and anti-static agents are applied to the
filament surface. Scouring before dyeing effectively removes these spin finishes.
Both nylon 6.6 and 6 are susceptible to degradation by ultraviolet light resulting
in yellowing and loss of strength. Lustrous filaments have better ultraviolet
resistance than those dulled by pigmentation with titanium dioxide. Ultraviolet
stabilisers, which protect the polymer by strongly absorbing ultraviolet light and
converting the energy into heat, are added to the nylon before spinning.
4.2.2 Production of nylon 6
Nylon 6 is manufactured similarly to nylon 6.6 by heating caprolactam with a little
water, a chain stopper such as acetic acid, and an acid catalyst, gradually removing