Page 69 - Basic Principles of Textile Coloration
P. 69
58 SYNTHETIC FIBRES
Nylons dyeable with cationic dyes, but which resist acid dyes, have a smaller
number of amino groups. They come from polymerisation of adipic acid with
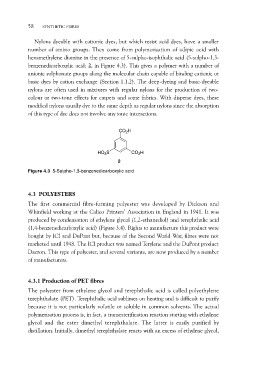
hexamethylene diamine in the presence of 5-sulpho-isophthalic acid (5-sulpho-1,3-
benzenedicarboxylic acid; 2, in Figure 4.3). This gives a polymer with a number of
anionic sulphonate groups along the molecular chain capable of binding cationic or
basic dyes by cation exchange (Section 1.1.2). The deep-dyeing and basic-dyeable
nylons are often used in mixtures with regular nylons for the production of two-
colour or two-tone effects for carpets and some fabrics. With disperse dyes, these
modified nylons usually dye to the same depth as regular nylons since the absorption
of this type of dye does not involve any ionic interactions.
CO2H
HO3S CO2H
2
Figure 4.3 5-Sulpho-1,3-benzenedicarboxylic acid
4.3 POLYESTERS
The first commercial fibre-forming polyester was developed by Dickson and
Whinfield working at the Calico Printers’ Association in England in 1941. It was
produced by condensation of ethylene glycol (1,2-ethanediol) and terephthalic acid
(1,4-benzenedicarboxylic acid) (Figure 3.4). Rights to manufacture this product were
bought by ICI and DuPont but, because of the Second World War, fibres were not
marketed until 1948. The ICI product was named Terylene and the DuPont product
Dacron. This type of polyester, and several variants, are now produced by a number
of manufacturers.
4.3.1 Production of PET fibres
The polyester from ethylene glycol and terephthalic acid is called polyethylene
terephthalate (PET). Terephthalic acid sublimes on heating and is difficult to purify
because it is not particularly volatile or soluble in common solvents. The actual
polymerisation process is, in fact, a transesterification reaction starting with ethylene
glycol and the ester dimethyl terephthalate. The latter is easily purified by
distillation. Initially, dimethyl terephthalate reacts with an excess of ethylene glycol,